LSD: Difference between revisions
m leave out some distracting detail on MKULTRA, par break, give date of prohibition in intro |
|||
| Line 33: | Line 33: | ||
LSD was first [[chemical synthesis|synthesized]] by [[Albert Hofmann]] in 1938 from [[ergot]], a [[grain]] [[fungus]] that typically grows on [[rye]]. The short form LSD comes from its early code name ''LSD-25'', which is an abbreviation for the German "Lysergsäure-diethylamid" followed by a sequential number.<ref name="problem-child">Hofmann, Albert. ''[http://www.psychedelic-library.org/child.htm LSD—My Problem Child]'' (McGraw-Hill, 1980). ISBN 0-07-029325-2.</ref><ref name="tihkal" /> LSD is sensitive to [[oxygen]], [[ultraviolet|ultraviolet light]], and [[chlorine]], especially in [[solution]], though its potency may last for years if it is stored away from light and moisture at low temperature. In pure form it is a colorless, odorless, and mildly bitter solid.<ref>{{cite book |title= [[PiHKAL]] |publisher= Transform Press |author1= Shulgin, Alexander |author2= Shulgin, Ann |year=1991 |edition=1st |chapter=Burt |page=21 |isbn= 978-0-9630096-0-9}}</ref> LSD is typically delivered orally, usually on a substrate such as absorbent [[Blotting paper|blotter paper]], a [[sugar|sugar cube]], or [[gelatin]]. In its liquid form, it can also be administered by intramuscular or intravenous injection. LSD is very potent, with 20–30 [[kilogram#SI multiples|µg]] (micrograms) being the threshold dose.<ref name="greiner" /> |
LSD was first [[chemical synthesis|synthesized]] by [[Albert Hofmann]] in 1938 from [[ergot]], a [[grain]] [[fungus]] that typically grows on [[rye]]. The short form LSD comes from its early code name ''LSD-25'', which is an abbreviation for the German "Lysergsäure-diethylamid" followed by a sequential number.<ref name="problem-child">Hofmann, Albert. ''[http://www.psychedelic-library.org/child.htm LSD—My Problem Child]'' (McGraw-Hill, 1980). ISBN 0-07-029325-2.</ref><ref name="tihkal" /> LSD is sensitive to [[oxygen]], [[ultraviolet|ultraviolet light]], and [[chlorine]], especially in [[solution]], though its potency may last for years if it is stored away from light and moisture at low temperature. In pure form it is a colorless, odorless, and mildly bitter solid.<ref>{{cite book |title= [[PiHKAL]] |publisher= Transform Press |author1= Shulgin, Alexander |author2= Shulgin, Ann |year=1991 |edition=1st |chapter=Burt |page=21 |isbn= 978-0-9630096-0-9}}</ref> LSD is typically delivered orally, usually on a substrate such as absorbent [[Blotting paper|blotter paper]], a [[sugar|sugar cube]], or [[gelatin]]. In its liquid form, it can also be administered by intramuscular or intravenous injection. LSD is very potent, with 20–30 [[kilogram#SI multiples|µg]] (micrograms) being the threshold dose.<ref name="greiner" /> |
||
Introduced by [[Sandoz Laboratories]], with trade-name '''Delysid''', as a drug with various [[psychiatric]] uses in 1947, LSD quickly became a [[psychedelic psychotherapy|therapeutic agent]] that appeared to show great promise. |
Introduced by [[Sandoz Laboratories]], with trade-name '''Delysid''', as a drug with various [[psychiatric]] uses in 1947, LSD quickly became a [[psychedelic psychotherapy|therapeutic agent]] that appeared to show great promise. In the [[USA]], a super-secretive [[CIA]] [[MKULTRA|research program into methods of behaviour-control outside the scope and ethics of legal civilian interaction]][http://www.gwu.edu/~nsarchiv/radiation/dir/mstreet/commeet/meet4/brief4.gfr/tab_j/br4j1.txt][http://www.saferchildren.net/print/senate01.pdf], lead directly to the drug being [http://history.howstuffworks.com/american-history/cia-lsd3.htm introduced] (via [[Ken Kesey]], 'founder' of the [[hippy movement]]) to the burgeoning [[youth culture]] in the [[Western world]] during the 1960s, later leading to a political firestorm that resulted in its US [[Psychedelics, dissociatives and deliriants#Legal status and attitudes|prohibition]] in 1965.<ref>{{cite web|url=http://www.rsc.org/chemistryworld/Issues/2006/January/LSD.asp |title=LSD: cultural revolution and medical advances |accessdate=2007-09-27 |work=Royal Society of Chemistry }}</ref> |
||
A number of organizations—including [[Beckley Foundation|the Beckley Foundation]], [[Multidisciplinary Association for Psychedelic Studies|MAPS]], [[Heffter Research Institute]] and the [[Albert Hofmann|Albert Hofmann Foundation]]—exist to fund, encourage and coordinate research into its medicinal uses.<ref>{{cite web|url=http://www.hofmann.org/ |title=The Albert Hofmann Foundation |accessdate=2007-09-27 |work=Hofmann Foundation }}</ref> |
|||
{{TOC limit|limit=4}} |
{{TOC limit|limit=4}} |
||
Revision as of 12:34, 9 April 2010
 | |
| Clinical data | |
|---|---|
| Other names | LSD, LSD-25, lysergide, D-lysergic acid diethyl amide, N,N- diethyl- D- lysergamide |
| Routes of administration | Oral, Intravenous |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Elimination half-life | 3–5 hours[1][2] |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.031 |
| Chemical and physical data | |
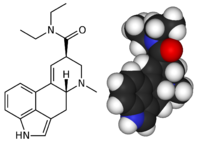
| Formula | C20H25N3O |
| Molar mass | 323.43 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 80 °C (176 °F) |
| |
| (verify) | |
Lysergic acid diethylamide, abbreviated LSD or LSD-25, also known as lysergide and colloquially as acid, is a semisynthetic psychedelic drug of the ergoline and tryptamine families. LSD is non-addictive, non-toxic, and is well known for its psychological effects which can include altered thinking processes, closed and open eye visuals, synaesthesia, a sense of time distortion, ego death and spiritual experiences, as well as for its key role in 1960s counterculture. It is used mainly by psychonauts as an entheogen, recreational drug and as an agent in psychedelic therapy.
LSD was first synthesized by Albert Hofmann in 1938 from ergot, a grain fungus that typically grows on rye. The short form LSD comes from its early code name LSD-25, which is an abbreviation for the German "Lysergsäure-diethylamid" followed by a sequential number.[3][4] LSD is sensitive to oxygen, ultraviolet light, and chlorine, especially in solution, though its potency may last for years if it is stored away from light and moisture at low temperature. In pure form it is a colorless, odorless, and mildly bitter solid.[5] LSD is typically delivered orally, usually on a substrate such as absorbent blotter paper, a sugar cube, or gelatin. In its liquid form, it can also be administered by intramuscular or intravenous injection. LSD is very potent, with 20–30 µg (micrograms) being the threshold dose.[6]
Introduced by Sandoz Laboratories, with trade-name Delysid, as a drug with various psychiatric uses in 1947, LSD quickly became a therapeutic agent that appeared to show great promise. In the USA, a super-secretive CIA research program into methods of behaviour-control outside the scope and ethics of legal civilian interaction[3][4], lead directly to the drug being introduced (via Ken Kesey, 'founder' of the hippy movement) to the burgeoning youth culture in the Western world during the 1960s, later leading to a political firestorm that resulted in its US prohibition in 1965.[7]
A number of organizations—including the Beckley Foundation, MAPS, Heffter Research Institute and the Albert Hofmann Foundation—exist to fund, encourage and coordinate research into its medicinal uses.[8]
Chemistry and structure

LSD is an ergoline derivative. It is commonly synthesised by reacting diethylamine with an activated form of lysergic acid. Activating reagents include phosphoryl chloride[9] and peptide coupling reagents.[10] Lysergic acid is made by alkaline hydrolysis of lysergamides like ergotamine, a substance derived from the ergot fungus on rye, or from ergine (lysergic acid amide, LSA), a compound that is found in morning glory (Ipomoea tricolor) and hawaiian baby woodrose (Argyreia nervosa) seeds.
LSD is a chiral compound with two stereocenters at the carbon atoms C-5 and C-8, so that theoretically four different optical isomers of LSD could exist. LSD, also called (+)-D-LSD, has the absolute configuration (5R,8R). The C-5 isomers of lysergamides do not exist in nature and are not formed during the synthesis from D-lysergic acid. Retrosynthetically, the C-5 chiral center could be analysed as having the same chirality of the alpha carbon of the biological amino acid L-tryptophan, the precursor to all biosynthetic ergoline compounds.
However, LSD and iso-LSD, the two C-8 isomers, rapidly interconvert in the presence of base, as the alpha proton is especially acidic and can be removed to catayse conversion to enol or enolate form. The enolate and enol forms are stabilised by resonance interactions with the aromatic indole ring. Non-psychoactive iso-LSD which has formed during the synthesis can be removed by chromatography and can be isomerized to LSD.
Though acid nominally also activates a carbonyl compound's enol form, LSD has a tertiary amine group and enamine-like system, both which compete as basic sites for protonation. Acid also triggers unwanted isomerisation of the ring-adjacent double bond via tertiary carbocation intermediate. (This isomerisation also eliminates the sensitive chiral center next to the tertiary amino group.) The carbocation is especially resonance-stabilised, as the carbocation position is next to an electron-donating indole ring. The presence of nucleophiles triggers a rich array of degradation pathways in the presence of acid.
A totally pure salt of LSD will emit small flashes of white light when shaken in the dark.[4] LSD is strongly fluorescent and will glow bluish-white under UV light.
Reactivity and degradation
"LSD," writes the chemist Alexander Shulgin, "is an unusually fragile molecule."[4] It is stable for indefinite time if stored as a solid salt or dissolved in water, at low temperature and protected from air and light exposure.
LSD has two sensitive chiral tertiary protons at the C5 and C8 positions that are prone to racemisation. The C8 proton is more labile due to the electron-withdrawing carboxamide attachment, but removal of the chiral proton at the C5 position (which actually was once also an alpha proton of the parent molecule tryptophan) is assisted by the inductively-withdrawing nitrogen and pi electron delocalisation with the indole ring.
LSD also has enamine-type reactivity because of the electron-donating effects of the indole ring. Because of this, chlorine destroys LSD molecules on contact; even though chlorinated tap water typically contains only a slight amount of chlorine, because a typical LSD solution only contains a small amount of LSD, dissolving LSD in tap water is likely to completely eliminate the substance.[4] The double bond between the 8-position and the aromatic ring, being conjugated with the indole ring, is susceptible to nucleophilic attacks by water or alcohol, especially in the presence of light. LSD often converts to "lumi-LSD", which is totally inactive in human beings (to the best of current knowledge).
A controlled study was undertaken to determine the stability of LSD in pooled urine samples.[11] The concentrations of LSD in urine samples were followed over time at various temperatures, in different types of storage containers, at various exposures to different wavelengths of light, and at varying pH values. These studies demonstrated no significant loss in LSD concentration at 25 °C for up to four weeks. After four weeks of incubation, a 30% loss in LSD concentration at 37 °C and up to a 40% at 45 °C were observed. Urine fortified with LSD and stored in amber glass or nontransparent polyethylene containers showed no change in concentration under any light conditions. Stability of LSD in transparent containers under light was dependent on the distance between the light source and the samples, the wavelength of light, exposure time, and the intensity of light. After prolonged exposure to heat in alkaline pH conditions, 10 to 15% of the parent LSD epimerized to iso-LSD. Under acidic conditions, less than 5% of the LSD was converted to iso-LSD. It was also demonstrated that trace amounts of metal ions in buffer or urine could catalyze the decomposition of LSD and that this process can be avoided by the addition of EDTA.
Dosage


A single dose of LSD may be between 100 and 500 micrograms — an amount roughly equal to one-tenth the mass of a grain of sand. Threshold effects can be felt with as little as 25 micrograms of LSD.[6][6][12] Dosages of LSD are measured in micrograms (µg), or millionths of a gram. By comparison, dosages of most drugs, both recreational and medicinal, are measured in milligrams (mg), or thousandths of a gram. For example, an active dose of mescaline, roughly 0.2 to 0.5g, has effects comparable to 100 µg or less of LSD.[3]
Typical doses in the 1960s ranged from 200 to 1000 µg while street samples of the 1970s contained 30 to 300 µg. By the 1980s, the amount had reduced to between 100 to 125 µg, lowering more in the 1990s to the 20–80 µg range.[13]
Estimates for the lethal dosage (LD50) of LSD range from between 200 µg/kg to more than 1 mg/kg of human body mass, though most sources report that there are no known human cases of such an overdose. Other sources note one report of a suspected fatal overdose of LSD occurring in November 1975 in Kentucky in which there were indications that ~1/3 of a gram (320 mg or 320,000 µg) had been injected intravenously. (This is a very extraordinary amount, particularly when compared to the average LSD dosage of ~100 µg).[14][15] Experiments with LSD have also been done on animals; in 1962, an elephant named Tusko died shortly after being injected with 297 mg, but whether the LSD was the cause of his death is controversial.[16]
History
LSD was first synthesized on November 16, 1938[17] by Swiss chemist Dr. Albert Hofmann at the Sandoz Laboratories in Basel, Switzerland as part of a large research program searching for medically useful ergot alkaloid derivatives. LSD's psychedelic properties were discovered 5 years later when Hofmann accidentally ingested an unknown quantity of the chemical.[18] The first intentional ingestion of LSD occurred on April 19, 1943, when Dr. Hofmann ingested 250 µg of LSD. He hypothesized this would be a threshold dose based on the dosages of other ergot alkaloids. Hofmann found the effects to be much stronger than he anticipated.[19] Sandoz Laboratories introduced LSD as a psychiatric drug in 1947.[20]
Beginning in the 1950s the US Central Intelligence Agency began a research program code named Project MKULTRA. Experiments included administering LSD to CIA employees, military personnel, doctors, other government agents, prostitutes, mentally ill patients, and members of the general public in order to study their reactions, usually without the subject's knowledge. The project was revealed in the US congressional Rockefeller Commission report in 1975.
In 1963 the Sandoz patents expired on LSD.[13] Also in 1963, the US Food and Drug Administration classified LSD as an Investigational New Drug, which meant new restrictions on medical and scientific use.[13] Several figures, including Aldous Huxley, Timothy Leary, and Al Hubbard, began to advocate the use of LSD. LSD became central to the counterculture of the 1960s. On October 24, 1968, possession of LSD was made illegal in the United States.[21] The last FDA approved human study with LSD, for use in dying cancer patients, ended in 1980. Legally approved and regulated psychiatric use of LSD continued in Switzerland until 1993.[22] Today, medical research is resuming around the world.[23]
Effects
Physical
LSD reliably causes pupil dilation, reduced appetite, and wakefulness. Other physical reactions to LSD are highly variable and nonspecific, and some of these reactions may be secondary to the psychological effects of LSD. The following symptoms have been reported: numbness, weakness, nausea, hypothermia or hyperthermia (decreased or increased body temperature), elevated blood sugar, goose bumps, increase in heart rate, jaw clenching, perspiration, saliva production, mucus production, sleeplessness, hyperreflexia, and tremors. Uterine contractions have been reported in animals.[citation needed] Some users, including Albert Hofmann, report a strong metallic taste for the duration of the effects. [24]
LSD is not considered addictive by the medical community.[25] Rapid tolerance build-up prevents regular use, and there is cross-tolerance shown between LSD, mescaline[26] and psilocybin.[27] This tolerance diminishes after a few days without use and is probably caused by downregulation of 5-HT2A receptors in the brain.[citation needed]
Psychological
LSD's psychological effects (colloquially called a "trip") vary greatly from person to person, depending on factors such as previous experiences, state of mind and environment, as well as dose strength. They also vary from one trip to another, and even as time passes during a single trip. An LSD trip can have long-term psychoemotional effects; some users cite the LSD experience as causing significant changes in their personality and life perspective. Widely different effects emerge based on what Timothy Leary called set and setting; the "set" being the general mindset of the user, and the "setting" being the physical and social environment in which the drug's effects are experienced.
Some psychological effects may include an experience of radiant colors, objects and surfaces appearing to ripple or "breathe," colored patterns behind the eyes, a sense of time distorting (time seems to be stretching, repeating itself, changing speed or stopping), crawling geometric patterns overlaying walls and other objects, morphing objects, a sense that one's thoughts are spiraling into themselves, loss of a sense of identity or the ego (known as "ego death"), and other powerful psycho-physical reactions.[28] Many users experience a dissolution between themselves and the "outside world".[29] This unitive quality may play a role in the spiritual and religious aspects of LSD. The drug sometimes leads to disintegration or restructuring of the user's historical personality and creates a mental state that some users report allows them to have more choice regarding the nature of their own personality.
If the user is in a hostile or otherwise unsettling environment, or is not mentally prepared for the powerful distortions in perception and thought that the drug causes, effects are more likely to be unpleasant than if he or she is in a comfortable environment and has a relaxed, balanced and open mindset.[30]
Sensory / perception
LSD causes expansion and an altered experience of senses, emotions, memories, time, and awareness for 6 to 14 hours, depending on dosage and tolerance. Generally beginning within thirty to ninety minutes after ingestion, the user may experience anything from subtle changes in perception to overwhelming cognitive shifts. Changes in auditory and visual perception are typical.[29][31] Visual effects include the illusion of movement of static surfaces ("walls breathing"), after image-like trails of moving objects ("tracers"), the appearance of moving colored geometric patterns (especially with closed eyes), an intensification of colors and brightness ("sparkling"), new textures on objects, blurred vision, and shape suggestibility. Users commonly report that the inanimate world appears to animate in an unexplained way; for instance, objects that are static in three dimensions can seem to be moving relative to one or more additional spatial dimensions.[32] Many of the basic visual effects resemble the phosphenes seen after applying pressure to the eye and have also been studied under the name "form constants". The auditory effects of LSD may include echo-like distortions of sounds, changes in ability to discern concurrent auditory stimuli, and a general intensification of the experience of music. Higher doses often cause intense and fundamental distortions of sensory perception such as synaesthesia, the experience of additional spatial or temporal dimensions, and temporary dissociation.
Potential use
LSD has been used in psychiatry for its perceived therapeutic value, in the treatment of alcoholism, pain and cluster headache relief, for spiritual purposes, and to enhance creativity. However, government organizations like the United States Drug Enforcement Administration maintain that LSD "produces no aphrodisiac effects, does not increase creativity, has no lasting positive effect in treating alcoholics or criminals, does not produce a 'model psychosis', and does not generate immediate personality change."[33]
Psychotherapy
In the 1950s and 1960s LSD was used in psychiatry to enhance psychotherapy. Some psychiatrists believed LSD was especially useful at helping patients to "unblock" repressed subconscious material through other psychotherapeutic methods,[34] and also for treating alcoholism. One study concluded, "The root of the therapeutic value of the LSD experience is its potential for producing self-acceptance and self-surrender,"[35] presumably by forcing the user to face issues and problems in that individual's psyche.
In December 1968, a survey was made of all 74 UK doctors who had used LSD in humans, 73 replied, 1 had moved overseas and was unavailable. Of the 73 replies, the majority of UK doctors with clinical experience with LSD felt that LSD was effective and had acceptable safety: 41 (56%) continued with clinical use of LSD, 11 (15%) had stopped because of retirement or other extraneous reasons, 9 (12%) had stopped because they found LSD ineffective, and 5 (7%) had stopped because they felt LSD was too dangerous.[36]
Alcoholism
Some studies in the 1950s that used LSD to treat alcoholism professed a 50% success rate,[37] five times higher than estimates near 10% for Alcoholics Anonymous.[38] These studies were criticized for methodological flaws, and different groups had inconsistent results. Mangini's 1998 paper reviewed this history. She concluded that the efficacy of LSD in treating alcoholism remains an open question.[39]
Pain
LSD was studied in the 1960s by Eric Kast as an analgesic for serious and chronic pain caused by cancer or other major trauma.[40] Even at low (sub-psychedelic) dosages, it was found to be at least as effective as traditional opiates, while being much longer lasting pain reduction (lasting as long as a week after peak effects had subsided). Kast attributed this effect to a decrease in anxiety. This reported effect is being tested (though not using LSD) in an ongoing (as of 2006) study of the effects of the psychedelic tryptamine psilocybin on anxiety in terminal cancer patients.
Cluster headaches
LSD has been used as a treatment for cluster headaches, an uncommon but extremely painful disorder. Researcher Peter Goadsby describes the headaches as "worse than natural childbirth or even amputation without anesthetic."[41] Although the phenomenon has not been formally investigated, case reports indicate that LSD and psilocybin can reduce cluster pain and also interrupt the cluster-headache cycle, preventing future headaches from occurring. Currently existing treatments include various ergolines, among other chemicals, so LSD's efficacy may not be surprising. A dose-response study testing the effectiveness of both LSD and psilocybin was planned at McLean Hospital, although the current status of this project is unclear. A 2006 study by McLean researchers interviewed 53 cluster-headache sufferers who treated themselves with either LSD or psilocybin, finding that a majority of the users of either drug reported beneficial effects.[42] Unlike use of LSD or MDMA in psychotherapy, this research involves non-psychological effects and often sub-psychedelic dosages.[43]
Spiritual
LSD is considered an entheogen because it can catalyze intense spiritual experiences, during which users may feel they have come into contact with a greater spiritual or cosmic order. Users claim to experience lucid sensations where they have "out of body" experiences. Some users report insights into the way the mind works, and some experience permanent shifts in their life perspective. Some users consider LSD a religious sacrament, or a powerful tool for access to the divine. Dr. Stanislav Grof has written that religious and mystical experiences observed during LSD sessions appear to be phenomenologically indistinguishable from similar descriptions in the sacred scriptures of the great religions of the world and the secret mystical texts of ancient civilizations.[44]
Creativity
In the 1950s and 1960s, psychiatrists like Oscar Janiger explored the potential effect of LSD on creativity. Experimental studies attempted to measure the effect of LSD on creative activity and aesthetic appreciation.[45][46][47][48] One patient of Dr. Janiger, bipolar and alcoholic artist Frank Murdoch,[49] was given a controlled, experimental dose of LSD for several months in an attempt to cure his late stage alcoholism. Janiger had Murdoch paint still-lives both on and off LSD, including a Kachina doll (that he reportedly had 70 other patients also paint).[50] The artists/ patients produced some 250 paintings and drawings after ingesting LSD).[50].
Dangers

LSD may temporarily impair the ability to make sensible judgments and understand common dangers, thus making the user more susceptible to accidents and personal injury and cause signs of organic brain damage-impaired memory and attention span, mental confusion or difficulty with abstract thinking.[53]
Adverse Drug Interactions
There is some indication that LSD may trigger a dissociative fugue state in individuals who are taking certain classes of antidepressants such as lithium salts and tricyclics. In such a state, the user has an impulse to wander, and may not be aware of his or her actions, which can lead to physical injury. Anonymous anecdotal reports have attributed seizures and one death to the combination of LSD with lithium.[54] SSRIs noticeably reduce LSD's subjective effects.[55] MAOIs are also reported to reduce the effects of LSD.[54]
Panic/Anxiety
LSD may elicit panic attacks or feelings of extreme anxiety.[56]
Psychosis
There are some cases of LSD inducing a psychosis in people who appeared to be healthy prior to taking LSD.[57] In most cases, the psychosis-like reaction is of short duration, but in other cases it may be chronic. It is difficult to determine whether LSD itself induces these reactions or if it triggers latent conditions that would have manifested themselves otherwise. The similarities of time course and outcomes between putatively LSD-precipitated and other psychoses suggest that the two types of syndromes are not different and that LSD may have been a nonspecific trigger.
Estimates of the prevalence of LSD-induced prolonged psychosis lasting over 48 hours have been made by surveying researchers and therapists who had administered LSD:
- Cohen (1960) estimated 0.8 per 1,000 volunteers (the single case among approximately 1250 study volunteers was the identical twin of a schizophrenic and he recovered within 5 days) and 1.8 per 1,000 psychiatric patients (7 cases among approximately 3850 patients, of which 2 cases were "preschizophrenic" or had previous hallucinatory experience, 1 case had unknown outcome, 1 case had incomplete recovery, and 5 cases recovered within up to 6 months).[58]
- Malleson (1971) reported no cases of psychosis among experimental subjects (170 volunteers who received a total of 450 LSD sessions) and estimated 9 per 1,000 among psychiatric patients (37 cases among 4300 patients, of which 8 details are unknown, 10 appeared chronic, and 19 recovered completely within up to 3 months).[36]
However, in neither survey study was it possible to compare the rate of lasting psychosis in these volunteers and patients receiving LSD with the rate of psychosis found in other groups of research volunteers or in other methods of psychiatric treatment (for example, those receiving placebo).
Cohen (1960) noted:[58]
- "The hallucinogenic experience is so striking that many subsequent disturbances may be attributed to it without further justification. The highly suggestible or hysterical individual would tend to focus on his LSD experience to explain subsequent illness. Patients have complained to Abramson that their LSD exposure produced migraine headaches and attacks of influenza up to a year later. One Chinese girl became paraplegic and ascribed that catastrophe to LSD. It so happened that these people were all in the control group and had received nothing but tap water."
Flashbacks and HPPD
"Flashbacks" are a reported psychological phenomenon in which an individual experiences an episode of some of LSD's subjective effects long after the drug has worn off, usually in the days after typical doses. In some rarer cases, flashbacks have lasted longer, but are generally short-lived and mild compared to the actual LSD "trip". Flashbacks can incorporate both positive and negative aspects of LSD trips, and are typically elicited by triggers such as alcohol or cannabis use, stress, or sleepiness. Flashbacks have proven difficult to study and are no longer officially recognized as a psychiatric syndrome. However, colloquial usage of the term persists and usually refers to any drug-free experience reminiscent of psychedelic drug effects, with the typical connotation that the episodes are of short duration.
No definitive explanation is currently available for these experiences. Any attempt at explanation must reflect several observations: first, over 70 percent of LSD users claim never to have "flashed back"; second, the phenomenon does appear linked with LSD use, though a causal connection has not been established; and third, a higher proportion of psychiatric patients report flashbacks than other users.[59] Several studies have tried to determine how likely a user of LSD, not suffering from known psychiatric conditions, is to experience flashbacks. The larger studies include Blumenfeld's in 1971[60] and Naditch and Fenwick's in 1977,[61] which arrived at figures of 20% and 28%, respectively.
Although flashbacks themselves are not recognized as a medical syndrome, there is a recognized syndrome called Hallucinogen Persisting Perception Disorder (HPPD) in which LSD-like visual changes are not temporary and brief, as they are in flash-backs, but instead are persistent, and cause clinically significant impairment or distress. The syndrome is a DSM-IV diagnosis. Several scientific journal articles have described the disorder.[62]
HPPD differs from flashbacks in that it is persistent and apparently entirely visual (although mood and anxiety disorders are sometimes diagnosed in the same individuals). A recent review suggests that HPPD (as defined in the DSM-IV) is rare and affects only a distinctly vulnerable subpopulation of users.[63] However, it is possible that the prevalence of HPPD is underestimated because most of the diagnoses are applied to people who are willing to admit to their health care practitioner that they have previously used psychotropics, and presumably many people are reluctant to admit this.[64]
There is no consensus regarding the nature and causes of HPPD (or flashbacks). A study of 44 HPPD subjects who had previously ingested LSD showed EEG abnormalities.[65] Given that some symptoms have environmental triggers, it may represent a failure to adjust visual processing to changing environmental conditions. There are no explanations for why only some individuals develop HPPD. Explanations in terms of LSD physically remaining in the body for months or years after consumption have been discounted by experimental evidence.[59] Some say HPPD is a manifestation of post-traumatic stress disorder, not related to the direct action of LSD on brain chemistry, and varies according to the susceptibility of the individual to the disorder. Many emotionally intense experiences can lead to flashbacks when a person is reminded acutely of the original experience. However, not all published case reports of HPPD appear to describe an anxious hyper-vigilant state reminiscent of post-traumatic stress disorder. Instead, some cases appear to involve only visual symptoms.[59]
Uterine contractions
Early pharmacological testing by Sandoz in laboratory animals showed that LSD can stimulate uterine contractions, with efficacy comparable to ergobasine, the active uterotonic component of the ergot fungus. (Hofmann's work on ergot derivatives also produced a modified form of ergobasine which became a widely accepted medication used in obstetrics, under the trade name Methergine.) Therefore, LSD use by pregnant women could be dangerous and is contraindicated.[3] However, the relevance of these animal studies to humans is unclear, and a 2008 medical reference guide to drugs in pregnancy and lactation stated, "It appears unlikely that pure LSD administered in a controlled condition is an abortifacient."[66]
Genetic
Beginning in 1967, studies raised concerns that LSD might produce genetic damage[67] or developmental abnormalities in fetuses. However, these initial reports were based on in vitro studies or were poorly controlled and have not been substantiated. In studies of chromosomal changes in human users and in monkeys, the balance of evidence suggests no increase in chromosomal damage. For example, white blood cells of people who had been given LSD in a clinical setting were examined for visible chromosomal abnormalities; overall, there appeared to be no lasting changes.[67] Several studies have been conducted using illicit LSD users and provide a less clear picture. Interpretation of this data is generally complicated by factors such as the unknown chemical composition of street LSD, concurrent use of other psychoactive drugs, and diseases such as hepatitis in the sampled populations. It seems possible that the small number of genetic abnormalities reported in users of street LSD is either coincidental or related to factors other than a toxic effect of pure LSD.[67] A 2008 medical review concluded, "The available data suggest that pure LSD does not cause chromosomal abnormalities, spontaneous abortions, or congenital malformations."[66]
"Antidotes"
Adverse effects of psychotropics are often treated with fast-acting benzodiazepines like diazepam or triazolam that have calming and antianxiety effects but do not directly affect the specific actions of psychotropics. Theoretically, specific 5-HT2A receptor antagonists, which most commonly means atypical antipsychotics (quetiapine, olanzapine, risperidone, etc.) or other 5-HT2A antagonist such as trazodone or mirtazapine, would be direct antidotes, although some anecdotal reports claim otherwise.[68] Also, some people have reported that taking an SSRI such as fluoxetine will counteract the effects of LSD.[69] Some reports indicate that although administration of chlorpromazine (Thorazine) or similar typical antipsychotic tranquilizers will not end an LSD trip, it will either lessen the intensity or immobilize and numb the patient, a side effect of the medication.[70] While it also may not end an LSD trip, the best chemical treatment for a "bad trip" is an anxiolytic agent such as diazepam (Valium) or another benzodiazepine. As the effect of the drug is psychological as well as physical, any treatment should focus on calming the patient. Limiting stimuli such as bright lights and loud noises can help in the event of an ill reaction.
Many rumors about home remedies to counteract psychedelic effects are circulated, including orange juice, vanilla essence, vitamin C, and anti-histamines. These may have a placebo effect, working by making the taker think they have done something to make it better.[71]
Pharmacokinetics
LSD's effects normally last from 6–12 hours depending on dosage, tolerance, body weight and age[4] The Sandoz prospectus for "Delysid" warned: "intermittent disturbances of affect may occasionally persist for several days."[3] Contrary to early reports and common belief, LSD effects do not last longer than the amount of time significant levels of the drug are present in the blood. Aghajanian and Bing (1964) found LSD had an elimination half-life of only 175 minutes.[1] However, using more accurate techniques, Papac and Foltz (1990) reported that 1 µg/kg oral LSD given to a single male volunteer had an apparent plasma half-life of 5.1 hours, with a peak plasma concentration of 5 ng/mL at 3 hours post-dose.[2]
Detection in biological fluids
LSD may be quantified in urine as part of a drug abuse testing program, in plasma or serum to confirm a diagnosis of poisoning in hospitalized victims or in whole blood to assist in a forensic investigation of a traffic or other criminal violation or a case of sudden death. Both the parent drug and its major metabolite are unstable in biofluids when exposed to light, heat or alkaline conditions and therefore specimens should be protected from light, stored at the lowest possible temperature and analyzed quickly to minimize losses.[72]
Pharmacodynamics

LSD affects a large number of the G protein coupled receptors, including all dopamine receptor subtypes, and all adrenoreceptor subtypes, as well as many others. LSD binds to most serotonin receptor subtypes except for 5-HT3 and 5-HT4. However, most of these receptors are affected at too low affinity to be sufficiently activated by the brain concentration of approximately 10–20 nM.[73] In humans, recreational doses of LSD can affect 5-HT1A, 5-HT2A, 5-HT2C, 5-HT5A, and 5-HT6 receptors.[1][74] 5-HT5B receptors, which are not present in humans, also have a high affinity for LSD.[75] The psychedelic effects of LSD are attributed to its strong partial agonist effects at 5-HT2A receptors as specific 5-HT2A agonists are psychedelics and largely 5-HT2A specific antagonists block the psychedelic activity of LSD.[73] Exactly how this produces the drug's effects is unknown, but it is thought that it works by increasing glutamate release in the cerebral cortex and therefore excitation in this area, specifically in layers IV and V.[76] LSD, like many other drugs, has been shown to activate DARPP-32-related pathways.[77]
Production

Because an active dose of LSD is very minute, a large number of doses can be synthesized from a comparatively small amount of raw material. Beginning with ergotamine tartrate, for example, one can manufacture roughly one kilogram of pure, crystalline LSD from five kilograms of the ergotamine salt. Five kilograms of LSD — 25 kilograms of ergotamine tartrate — could provide 100 million doses, according to the DEA, more than enough to meet what is believed to be the entire annual U.S. demand. Since the masses involved are so small, concealing and transporting illicit LSD is much easier than smuggling other illegal drugs like cocaine or cannabis.[78]
Manufacturing LSD requires laboratory equipment and experience in the field of organic chemistry. It takes two to three days to produce 30 to 100 grams of pure compound. It is believed that LSD is not usually produced in large quantities, but rather in a series of small batches. This technique minimizes the loss of precursor chemicals in case a step does not work as expected.[78]
Forms of LSD

LSD is produced in crystalline form and then mixed with excipients or redissolved for production in ingestible forms. Liquid solution is either distributed in small vials or, more commonly, sprayed onto or soaked into a distribution medium. Historically, LSD solutions were first sold on sugar cubes, but practical considerations forced a change to tablet form. Appearing in 1968 as an orange tablet measuring about 6 mm across "Sunshine" acid was the first largely available form of LSD after its possession was made illegal. Tim Scully, a prominent chemist, made some of it, but said that most "Sunshine" in the USA came by way of Ronald Stark, who imported approximately thirty-five million doses from Europe.[79]
Over a period of time, tablet dimensions, weight, shape and concentration of LSD evolved from large (4.5-8.1 mm diameter), heavyweight (≥150 mg), round, high concentration (90-350 µg/tab) dosage units to small (2.0-3.5 mm diameter) lightweight (as low as 4.7 mg/tab), variously shaped, lower concentration (12-85 µg/tab, average range 30-40 µg/tab) dosage units. LSD tablet shapes have included cylinders, cones, stars, spacecraft and heart shapes. The smallest tablets became known as "Microdots".[80]
After tablets came "computer acid" or "blotter paper LSD," typically made by dipping a preprinted sheet of blotting paper into an LSD/water/alcohol solution.[79][80] More than 200 types of LSD tablets have been encountered since 1969 and more than 350 blotter paper designs have been observed since 1975.[80] About the same time as blotter paper LSD came "Windowpane" (aka "Clearlight"), which contained LSD inside a thin gelatin square a quarter of an inch across.[79] LSD has been sold under a wide variety of often short-lived and regionally restricted street names including Acid, Trips, Uncle Sid, Blotter, Lucy, and doses, as well as names that reflect the designs on the sheets of blotter paper.[81][82] Authorities have encountered the drug in other forms — including powder or crystal, and capsule.[83]
Modern distribution
LSD manufacturers and traffickers in the United States can be categorized into two groups: A few large-scale producers, and an equally limited number of small, clandestine chemists, consisting of independent producers who, operating on a comparatively limited scale, can be found throughout the country.[84] As a group, independent producers are of less concern to the Drug Enforcement Administration than the larger groups, as their product reaches only local markets.[85]
LSD Mimics


In recent years, law enforcement in the United States and elsewhere has seized several chemicals and combinations of chemicals in blotter paper which were sold as LSD mimics, including DOB,[86][87] 2C-I,[88][89] DOC,[89] a mixture of DOC and DOI,[90] and a mixture of DOC and DOB.[91] Street users of LSD are often under the impression that blotter paper which is actively hallucinogenic can only be LSD because that is the only chemical with low enough doses to fit on a small square of blotter paper. While it is true that LSD requires lower doses than most other hallucinogens, blotter paper is capable of absorbing a much larger amount of material. The DEA performed a chromatographic analysis of blotter paper containing 2C-C which showed that the paper contained a much greater concentration of the active chemical than typical LSD doses, although the exact quantity was not determined.[92] Blotter LSD mimics can have relatively small dose squares; a sample of blotter paper containing DOC seized by Concord, California police had dose markings approximately 6 mm apart.[89]
Legal status
The United Nations Convention on Psychotropic Substances (adopted in 1971) requires its parties to prohibit LSD. Hence, it is illegal in all parties to the convention, which includes the United States, Australia, New Zealand, and most of Europe. However, enforcement of extant laws varies from country to country. Medical and scientific research with LSD in humans is permitted under the 1971 UN Convention.
Canada
In Canada, LSD is a controlled substance under Schedule III of the Controlled Drugs and Substances Act.[93] Every person who seeks to obtain the substance, without disclosing authorization to obtain such substances 30 days prior to obtaining another prescription from a practitioner, is guilty of an indictable offense and liable to imprisonment for a term not exceeding 3 years. Possession for purpose of trafficking is an indictable offense punishable by imprisonment for 10 years.
United Kingdom
In the United Kingdom, LSD is a class A drug. This means that possession of the drug without a license is punishable with 7 years imprisonment and/or an unlimited fine, and trafficking is punishable with life imprisonment and an unlimited fine (see main article on drug punishments Misuse of Drugs Act 1971).
In 2000, after consultation with members of the Royal College of Psychiatrists' Faculty of Substance Misuse, the UK Police Foundation issued the Runciman Report which recommended "the transfer of LSD from Class A to Class B".[94]
In Nov 2009, the UK Transform Drug Policy Foundation released in the House of Commons a guidebooks to the legal regulation of drugs, After the War on Drugs: Blueprint for Regulation, which details options for regulated distribution and sale of LSD and other psychedelics.[95]
United States
LSD is Schedule I in the United States, according to the Controlled Substances Act of 1970.[96] This means LSD is illegal to manufacture, buy, possess, process, or distribute without a DEA license. By classifying LSD as a Schedule I substance, the Drug Enforcement Administration holds that LSD meets the following three criteria: it is deemed to have a high potential for abuse; it has no legitimate medical use in treatment; and there is a lack of accepted safety for its use under medical supervision. Note, there are very few or no documented deaths due to chemical toxicity, most LSD deaths are a result of behavioral toxicity.
There can also be substantial discrepancies between the amount of chemical LSD that one possesses and the amount of possession with which one can be charged in the U.S. This is because LSD is almost always present in a medium (e.g. blotter or neutral liquid), and the amount that can be considered with respect to sentencing is the total mass of the drug and its medium. This discrepancy was the subject of 1995 United States Supreme Court case, Neal v. U.S.[97]
Lysergic acid and lysergic acid amide, LSD precursors, are both classified in Schedule III of the Controlled Substances Act. Ergotamine tartrate, a precursor to lysergic acid, is regulated under the Chemical Diversion and Trafficking Act.
Notable individuals
Many notable individuals have commented publicly on their experiences with LSD.[98] Some of these comments date from the era when it was legally available in the US and Europe for non-medical uses, and others pertain to psychiatric treatment in the 1950s and 60s. Still others describe experiences with illegal LSD, obtained for philosophic, artistic, therapeutic, spiritual, or recreational purposes.
See also
- ALD-52, chemical analogue of LSD
- LSA, chemical analogue of LSD
- Methysergide, headache medication, chemically related to LSD
- Psychedelic drug
- Psychedelic experience
- Urban legends about LSD
References
- ^ a b c Aghajanian, George K.; Bing, Oscar H. L. (1964). "Persistence of lysergic acid diethylamide in the plasma of human subjects" (PDF). Clinical Pharmacology and Therapeutics. 5: 611–614. PMID 14209776. Retrieved 2009-09-17.
- ^ a b
Papac, DI; Foltz, RL (1990). "Measurement of lysergic acid diethylamide (LSD) in human plasma by gas chromatography/negative ion chemical ionization mass spectrometry" (PDF). Journal of Analytical Toxicology. 14 (3): 189–190. PMID 2374410. Retrieved 2009-09-17.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c d Hofmann, Albert. LSD—My Problem Child (McGraw-Hill, 1980). ISBN 0-07-029325-2.
- ^ a b c d e Alexander and Ann Shulgin. "LSD", in TiHKAL (Berkeley: Transform Press, 1997). ISBN 0-963-00969-9.
- ^ Shulgin, Alexander; Shulgin, Ann (1991). "Burt". PiHKAL (1st ed.). Transform Press. p. 21. ISBN 978-0-9630096-0-9.
- ^ a b c Greiner T, Burch NR, Edelberg R (1958). "Psychopathology and psychophysiology of minimal LSD-25 dosage; a preliminary dosage-response spectrum". AMA Arch Neurol Psychiatry. 79 (2): 208–10. PMID 13497365.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "LSD: cultural revolution and medical advances". Royal Society of Chemistry. Retrieved 2007-09-27.
- ^ "The Albert Hofmann Foundation". Hofmann Foundation. Retrieved 2007-09-27.
- ^ Monte AP, Marona-Lewicka D, Kanthasamy A, Sanders-Bush E, Nichols DE (1995). "Stereoselective LSD-like activity in a series of d-lysergic acid amides of (R)- and (S)-2-aminoalkanes". J. Med. Chem. 38 (6): 958–66. doi:10.1021/jm00006a015. PMID 7699712.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Nichols DE, Frescas S, Marona-Lewicka D, Kurrasch-Orbaugh DM (2002). "Lysergamides of isomeric 2,4-dimethylazetidines map the binding orientation of the diethylamide moiety in the potent hallucinogenic agent N,N-diethyllysergamide (LSD)". J. Med. Chem. 45 (19): 4344–9. doi:10.1021/jm020153s. PMID 12213075.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Li Z., McNally A. J., Wang H., Salamone S. J. (1998). "Stability study of LSD under various storage conditions" ([dead link]). J. Anal. Toxicol. 22 (6): 520–5. PMID 9788528.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Stoll, W.A. (1947). Ein neues, in sehr kleinen Mengen wirsames Phantastikum. Schweiz. Arch. Neur. 60,483.
- ^ a b c Henderson, Leigh A.; Glass, William J. (1994). LSD: Still with us after all these years. San Francisco: Jossey-Bass. ISBN 978-0787943790.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ "LSD Vault: Dosage". 2006-07-06. Retrieved 2007-01-31.
- ^ "LSD Toxicity: A Suspected Cause of Death". Journal of the Kentucky Medical Association. Retrieved 2007-11-26.
- ^ Erowid & R. Stuart (2002). "LSD Related Death of an Elephant - Controversy surrounding the 1962 death of an elephant after an injection of LSD". Erowid. Retrieved 2008-07-28.
- ^ Dr. Albert Hofmann; translated from the original German (LSD Ganz Persönlich) by J. Ott. MAPS-Volume 6 Number 69 Summer 1969
- ^ Nichols, David (2003-05-24). "Hypothesis on Albert Hofmann's Famous 1943 "Bicycle Day"". Hofmann Foundation. Retrieved 2007-09-27.
- ^ Hofmann, Albert. "History Of LSD". Retrieved 2007-09-27.
- ^ LSD: The Drug
- ^ United States Congress (1968-10-24). "Staggers-Dodd Bill, Public Law 90-639" (PDF). Retrieved 2009-09-08.
- ^ Gasser, Peter (1994). "Psycholytic Therapy with MDMA and LSD in Switzerland". Retrieved 2009-09-08.
- ^ MAPS Psychedelic Research
- ^ Albert Hofmann. "LSD: My Problem Child".
taste of metal on the palate
- ^ Lüscher C, Ungless MA (2006). ["http://www.plosmedicine.org/article/info:doi/10.1371/journal.pmed.0030437" "The mechanistic classification of addictive drugs"]. PLoS Med. 3 (11): e437. doi:10.1371/journal.pmed.0030437. PMC 1635740. PMID 17105338.
{{cite journal}}: Check|url=value (help); Unknown parameter|month=ignored (help)CS1 maint: unflagged free DOI (link) - ^ Wolbach AB Jr, Isbell H, Miner EJ (1962). "Cross tolerance between mescaline and LSD-25, with a comparison of the mescaline and LSD reactions". Psychopharmacologia. 3: 1–14. doi:10.1007/BF00413101. PMID 14007904.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Isbell H, Wolbach AB, Wikler A, Miner EJ (1961). "Cross Tolerance between LSD and Psilocybin". Psychopharmacologia. 2: E353. doi:10.1007/BF00407974. PMID 13717955.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ The Good Drugs Guide. "LSD psychedelic effects". Retrieved 2008-03-03.
- ^ a b Linton, Harriet B. and Langs, Robert J. "Subjective Reactions to Lysergic Acid Diethylamide (LSD-25)". Arch. Gen. Psychiat. Vol. 6 (1962): 352–68.
- ^ "LSD dangers". The Good Drugs Guide. Retrieved 2008-10-20.
- ^ Katz MM, Waskow IE, Olsson J (1968). "Characterizing the psychological state produced by LSD". J Abnorm Psychol. 73 (1): 1–14. doi:10.1037/h0020114. PMID 5639999.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ See, e.g., Gerald Oster's article "Moiré patterns and visual hallucinations". Psychedelic Rev. No. 7 (1966): 33–40.
- ^ DEA - Publications - LSD in the US - The Drug
- ^ Cohen, S. (1959). The therapeutic potential of LSD-25. A Pharmacologic Approach to the Study of the Mind, p251–258.
- ^ Chwelos N, Blewett D.B., Smith C.M., Hoffer A. (1959). "Use of d-lysergic acid diethylamide in the treatment of alcoholism". Quart. J. Stud. Alcohol. 20: 577–90. PMID 13810249.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Malleson, Nicholas (1971). "Acute Adverse Reactions to LSD in Clinical and Experimental Use in the United Kingdom" (PDF). Br J Psychiatry. 118 (543): 229–30. doi:10.1192/bjp.118.543.229. PMID 4995932.
- ^ Maclean, J.R.; Macdonald, D.C.; Ogden, F.; Wilby, E., "LSD-25 and mescaline as therapeutic adjuvants." In: Abramson, H., Ed., The Use of LSD in Psychotherapy and Alcoholism, Bobbs-Merrill: New York, 1967, pp. 407–426; Ditman, K.S.; Bailey, J.J., "Evaluating LSD as a psychotherapeutic agent," pp.74–80; Hoffer, A., "A program for the treatment of alcoholism: LSD, malvaria, and nicotinic acid," pp. 353–402.
- ^ Minogue SJ (1948). "Alcoholics Anonymous". Med. J. Aust. 1 (19): 586. PMID 18868217.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Mangini M (1998). "Treatment of alcoholism using psychedelic drugs: a review of the program of research". J Psychoactive Drugs. 30 (4): 381–418. PMID 9924844.
- ^ Kast, Eric (1967). "Attenuation of anticipation: a therapeutic use of lysergic acid diethylamide" (PDF). Psychiat. Quart. 41 (4): 646–57. doi:10.1007/BF01575629. PMID 4169685.
- ^ Dr. Goadsby is quoted in "Research into psilocybin and LSD as cluster headache treatment", and he makes an equivalent statement in an Health Report interview on Australian Radio National (August 9, 1999). Pages accessed 2007-01-31.
- ^ Sewell, R. A. (2006-06-27). "Response of cluster headache to psilocybin and LSD". Neurology. 66 (12): 1920–2. doi:10.1212/01.wnl.0000219761.05466.43. PMID 16801660.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Summarized from "Research into psilocybin and LSD as cluster headache treatment" and the Clusterbusters website. Pages accessed 2007-01-31.
- ^ Grof, Stanislav (1979). Realms of the Human Unconscious (Observations from LSD Research). London: Souvenir Press (E & A) Ltd. pp. 13–14. ISBN 0 285 64882 9.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Sessa, B. (2008). "Is it time to revisit the role of psychedelic drugs in enhancing human creativity?". J Psychopharmacol. 22 (8): 821–7. doi:10.1177/0269881108091597. PMID 18562421.
{{cite journal}}: Cite has empty unknown parameter:|coauthors=(help) - ^ Janiger, O. (1989). "LSD and creativity". J Psychoactive Drugs. 21 (1): 129–34. PMID 2723891.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Stafford, Peter G. (1967). LSD, the problem-solving psychedelic.
{{cite book}}: Cite has empty unknown parameter:|unused_data=(help); Text "ASIN: B0006BPSA0" ignored (help) - ^ McGlothlin, W. (1967). "Long lasting effects of LSD on normals" (PDF). Arch Gen Psychiat. 17 (5): 521–532. PMID 6054248.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Lynn Svensson (2006). Looking For Frank Murdoch: The LSD Experiments.
{{cite book}}: Text "http://" ignored (help) - ^ a b http://fusionanomaly.net/oscarjaniger.html Oscar Janiger
- ^ Fish, Jefferson M. (2006). Drugs and Society: U.S. Public Policy. Lanham, MD: Rowman & Littlefield. pp. 149–162. ISBN 0742542459.
- ^ http://web.cgu.edu/faculty/gabler/drug_toxicity.htm
- ^ LSD AND ORGANIC BRAIN IMPAIRMENT S Cohen, AE Edwards - Drug dependence, 1969
- ^ a b "LSD and Antidepressants" (2003) via Erowid.
- ^ Kit Bonson, "The Interactions between Hallucinogens and Antidepressants" (2006).
- ^ www.erowid.org/chemicalse/lsd/lsd_heath3.shtml
- ^ [Rick Strassman (1984). "Adverse reactions to psychedelic drugs. A review of the literature". J Nerv Ment Dis. 172 (10): 577–95. PMID 6384428.
{{cite journal}}: Cite has empty unknown parameter:|unused_data=(help); Text "Strassman RJ]" ignored (help) - ^ a b Cohen, Sidney (1960). "Lysergic Acid Diethylamide: Side Effects and Complications" (PDF). Journal of Nervous and Mental Disease. 130 (1): 30–40. PMID 13811003.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c David Abrahart (1998). "A Critical Review of Theories and Research Concerning Lysergic Acid Diethylamide (LSD) and Mental Health". Retrieved 2007-02-02.
- ^ Blumenfield M (1971). "Flashback phenomena in basic trainees who enter the US Air Force". Military Medicine. 136 (1): 39–41. PMID 5005369.
- ^ Naditch MP, Fenwick S (1977). "LSD flashbacks and ego functioning". Journal of Abnormal Psychology. 86 (4): 352–9. doi:10.1037/0021-843X.86.4.352. PMID 757972.
- ^ See, for example, Abraham HD, Aldridge AM (1993). "Adverse consequences of lysergic acid diethylamide". Addiction. 88 (10): 1327–34. doi:10.1111/j.1360-0443.1993.tb02018.x. PMID 8251869.
- ^ Halpern JH, Pope HG Jr (2003). "Hallucinogen persisting perception disorder: what do we know after 50 years?". Drug Alcohol Depend. 69 (2): 109–19. doi:10.1016/S0376-8716(02)00306-X. PMID 12609692.; Halpern JH (2003). "Hallucinogens: an update". Curr Psychiatry Rep. 5 (5): 347–54. doi:10.1007/s11920-003-0067-4. PMID 13678554. [1]
- ^ Baggott; et al. (2006). "Prevalence of chronic flashbacks in hallucinogen users: a web-based questionnaire" (PDF). Retrieved 2009-09-25.
{{cite web}}: Explicit use of et al. in:|author=(help) - ^ Abraham, H. D., & Duffy, F. H. ((1996)). "Stable quantitative EEG difference in post-LSD visual disorder by split-half analysis: Evidence for disinhibition". Psychiatry Research. 67 (3): 173–187. doi:10.1016/0925-4927(96)02833-8. PMID 8912957.
{{cite journal}}: Check date values in:|year=(help)CS1 maint: multiple names: authors list (link) - ^ a b Gerald G. Briggs,Roger K. Freeman,Sumner J. Yaffe (2008). Drugs in pregnancy and lactation. Lippincott Williams & Wilkins.
{{cite book}}: Unknown parameter|isnb=ignored (|isbn=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ a b c Dishotsky NI, Loughman WD, Mogar RE, Lipscomb WR (1971). "LSD and genetic damage" (PDF). Science. 172 (982): 431–40. doi:10.1126/science.172.3982.431. PMID 4994465.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Huxley, Aldous The Doors of Perception and Heaven & Hell, Harper & Row, 1954.
- ^ Bonson, Kit (1994). "The Interactions between Hallucinogens and Antidepressants - Summary of Results from Online Survey and Online Interviews". Erowid. Retrieved 2008-07-28.
- ^ Gilberti, F. and Gregoretti, L. L. (1955). "Prime esperienze di antaonismo psicofarmacologico" (PDF). Sistema Nervoso. 4: 301–309.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ RaveSafe (1999). "RaveSafe Q&A - Can you actually end an acid trip?". Retrieved 2008-07-28.
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 871-874.
- ^ a b Nichols, David E. (2004). "Psychotropics". Pharmacology & Therapeutics. 101 (2): 131–81. doi:10.1016/j.pharmthera.2003.11.002. PMID 14761703.
- ^ "PDSP database". Retrieved 2009-11-02.
- ^ Nelson, DL (2004). "5-HT5 receptors". Current drug targets. CNS and neurological disorders. 3 (1): 53–8. doi:10.2174/1568007043482606. PMID 14965244.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ BilZ0r. "The Neuropharmacology of Hallucinogens: a technical overview". Erowid, v3.1 (August 2005).
- ^ Svenningsson P. , Nairn A. C., Greengard P. (2005). "DARPP-32 Mediates the Actions of Multiple Drugs of Abuse". AAPS Journal. 07 (02): E353–E360. doi:10.1208/aapsj070235.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b DEA (2007). "LSD Manufacture - Illegal LSD Production". LSD in the United States. U.S. Department of Justice Drug Enforcement Administration. Archived from the original on 2007-08-29.
- ^ a b c Stafford, Peter (1992). "Chapter 1 - The LSD Family". Psychedelics Encyclopaedia (Third Expanded ed.). Ronin Publishing Inc. p. 62. ISBN 0-914171-51-8.
- ^ a b c Laing, Richard R. (2003). "Chapter 2.2 - Forms of the Drug". Hallucinogens: A Forensic Drug Handbook. Academic Press. pp. 39–41. ISBN 0124339514.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Honig, David. Frequently Asked Questions via Erowid
- ^ "Street Terms: Drugs and the Drug Trade". Office of National Drug Control Policy. 2005-04-05. Retrieved 2007-01-31.
- ^ DEA (2008). "Photo Library (page 2)". US Drug Enforcement Administration. Retrieved 2008-06-27.
- ^ ^ Maclean, J.R.; Macdonald, D.C.; Ogden, F.; Wilby, E., "LSD-25 and mescaline as therapeutic adjuvants." In: Abramson, H., Ed., The Use of LSD in Psychotherapy and Alcoholism, Bobbs-Merrill: New York, 1967, pp. 407–426; Ditman, K.S.; Bailey, J.J., "Evaluating LSD as a psychotherapeutic agent," pp.74–80; Hoffer, A., "A program for the treatment of alcoholism: LSD, malvaria, and nicotinic acid," pp. 353–402.
- ^ ^ LSD: The Drug
- ^
United States Drug Enforcement Administration (October 2005). Microgram Bulletin. 38 (10) http://www.usdoj.gov/dea/programs/forensicsci/microgram/mg1005/mg1005.html. Retrieved 2009-08-20.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: date and year (link) - ^
United States Drug Enforcement Administration (November 2006). Microgram Bulletin. 39 (11) http://www.usdoj.gov/dea/programs/forensicsci/microgram/mg1106/mg1106.html. Retrieved 2009-08-20.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: date and year (link) - ^
United States Drug Enforcement Administration (February 2007). Microgram Bulletin. 40 (2) http://www.usdoj.gov/dea/programs/forensicsci/microgram/mg0207/mg0207.html. Retrieved 2009-08-20.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: date and year (link) - ^ a b c
United States Drug Enforcement Administration (December 2007). Microgram Bulletin. 40 (12) http://www.usdoj.gov/dea/programs/forensicsci/microgram/mg1207/mg1207.html. Retrieved 2009-08-20.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: date and year (link) - ^
United States Drug Enforcement Administration (March 2008). Microgram Bulletin. 41 (3) http://www.usdoj.gov/dea/programs/forensicsci/microgram/mg0308/mg0308.html. Retrieved 2009-08-20.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: date and year (link) - ^
United States Drug Enforcement Administration (March 2009). Microgram Bulletin. 42 (3) http://www.usdoj.gov/dea/programs/forensicsci/microgram/mg0309/mg0309.html. Retrieved 2009-08-20.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: date and year (link) - ^
United States Drug Enforcement Administration (November 2005). Microgram Bulletin. 38 (11) http://www.usdoj.gov/dea/programs/forensicsci/microgram/mg1105/mg1105.html. Retrieved 2009-08-20.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: date and year (link) - ^ Canadian government (1996). "Controlled Drugs and Substances Act". Justice Laws. Canadian Department of Justice. Retrieved 2007-07-05.
- ^ Drugs and the law: Report of the inquiry into the Misuse of Drugs Act 1971 London: Police Foundation, 2000, Runciman Report
- ^ After the War on Drugs: Blueprint for Regulation Transform Drug Policy Foundation 2009
- ^ From [2]: LSD is a Schedule I substance under the Controlled Substances Act.
- ^ Neal v. United States, U.S. 284 (1996)., originating from U.S. v. Neal, 46 F.3d 1405 (7th Cir. 1995)
- ^ "Famous LSD users". The Good Drugs Guide. Retrieved 2008-10-20.
Further reading
- Bebergal, Peter, "Will Harvard drop acid again? Psychedelic research returns to Crimsonland", The Phoenix (Boston), June 2, 2008
- Grof, Stanislav. LSD Psychotherapy. (April 10, 2001)
- Lee, Martin A. and Bruce Shlain. Acid Dreams: The Complete Social History of LSD: The CIA, the Sixties, and Beyond (1992) ISBN 9780802130624
- Marks, John. The Search for the Manchurian Candidate: The CIA and Mind Control (1979), ISBN 0812907736
- Roberts, Andy. Albion Dreaming: A Popular History of LSD in Britain (2008), Marshall Cavendish,U.K, ISBN 1905736274
- Stevens, Jay. Storming Heaven: LSD And The American Dream (1998) ISBN 9780802135872
- Hofmann, Albert. LSD My Problem Child: Reflections on Sacred Drugs, Mysticism and Science (1983) ISBN 9780966001983
- Henderson, Leigh A. and William J. Glass. LSD: Still With Us After All These Years: Based on the National Institute of Drug Abuse Studies on the Resurgence of Contemporary LSD Use (1st edition 1994, 2nd edition 1998) ISBN 9780787943790
- de Rios, Marlene Dobkin and Oscar Janiger. LSD, spirituality, and the creative process (2003, Inner Traditions) ISBN 9780892819737 - "An exploration of how LSD influences imagination and the creative process. Based on the results of one of the longest clinical studies of LSD that took place between 1954 and 1962, before LSD was illegal. Includes personal reports, artwork, and poetry from the original sessions as testimony of the impact of LSD on the creative process."
- Passie T, Halpern JH, Stichtenoth DO, Emrich HM, Hintzen A. The pharmacology of lysergic acid diethylamide: a review. CNS Neurosci Ther. 2008 Winter;14(4):295-314. PMID 19040555
External links
- Erowid Vaults: LSD-25
- The Lycaeum Archive: LSD
- TIHKAL entry for LSD-25
- LSD entry in TiHKAL • info
- WWW Psychedelic Bibliography, MAPS - large database of scientific publications on LSD and other psychedelics, fulltext PDFs
- Current LSD Research – MAPS
- InfoFacts - Hallucinogens NIDA
- Talk to Frank UK government anti-drug site on LSD
- Scholarly bibliography on the histories of LSD use
- My LSD Trip: a non-cop, non-hippie report of the unvarnished facts, by Robert Gannon, Popular Science Magazine, December 1967.
- LSD Returns--For Psychotherapeutics (Scientific American Magazine article)
Documentaries
- Hofmann's Potion a documentary on the origins of LSD
- Power & Control LSD in The Sixties, documentary film directed by Aron Ranen, 2006
- Inside LSD National Geographic Channel, 2009
