Pentaerythritol tetranitrate: Difference between revisions
Provided a reference found on the PETN page as food for the Right Honorable and lazy Mr. Binksternet |
→References: ref format and -orphan |
||
| Line 52: | Line 52: | ||
==References== |
==References== |
||
{{reflist|2}} |
|||
* Cooper, Paul W., ''Explosives Engineering'', New York: Wiley-VCH, 1996. ISBN 0-471-18636-8 |
|||
<references/> |
|||
==External links== |
==External links== |
||
Revision as of 18:35, 22 April 2009
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ECHA InfoCard | 100.000.987 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C5H8N4O12 | |
| Molar mass | 316.137 g/mol |
| Density | 1.77 g/cm3 at 20 °C |
| Melting point | 141.3 °C (286.3 °F; 414.4 K) |
| Explosive data | |
| Shock sensitivity | Medium |
| Friction sensitivity | Medium |
| RE factor | 1.66 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
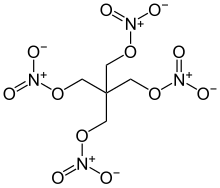
Pentaerythritol tetranitrate (PETN, also known as corpent, pentrite, or rarely and primarily in German as nitropenta or pentrit) [1] is one of the most powerful high explosives known, with a relative effectiveness factor (R.E. factor) of 1.66.
Uses
The most obvious use of PETN is as an explosive. It is more sensitive to shock or friction than TNT or tetryl, and it is never used alone as a booster. It is primarily used in booster and bursting charges of small caliber ammunition, in upper charges of detonators in some land mines and shells, and as the explosive core of detonation cord. [2] Apart from this, PETN is used as a vasodilator, similar to nitroglycerin. A medicine for heart disease, Lentonitrat, is nearly pure PETN.[3]
Production
PETN's preparation involves the nitration of pentaerythritol with a mixture of concentrated nitric and sulfuric acid. The preferred method of nitration is the ICI method, which utilizes concentrated nitric acid (98%+) alone, as mixed acid can create unstable sulfonated by-products.
C(CH2OH)4 + 4HNO3 → C(CH2ONO2)4 + 4H2O
History
Penthrite was first synthesized in 1891 by Tollens and Wiegand by nitration of pentaerythritol. In 1912, after being patented by the German government, the production of PETN started. PETN was used by the German army in World War I. [4] PETN is also one of the ingredients in Semtex plastic explosive. PETN was the explosive chosen by Richard Reid, which could have been used to blow up a 767 traveling from Paris to Miami if he wasn't stopped first.[5]
See also
References
- ^ PETN Synonyms, accessed 2009-02-19
- ^ * "Primacord Technical Information" (PDF). Dyno Nobel.
- ^ Russek H. I. (1966). "The therapeutic role of coronary vasodilators: glyceryl trinitrate, isosorbide dinitrate, and pentaerythritol tetranitrate". American Journal of Medical Science. 252 (1): 9–20. doi:10.1097/00000441-196607000-00002. PMID 4957459.
- ^ Stettbacher, Alfred (1933). Die Schiess- und Sprengstoffe. Leipzig: Barth. p. 459.
{{cite book}}: Cite has empty unknown parameter:|unused_data=(help); Text "2. völlig umgearb. Aufl." ignored (help) - ^ [1] Shoe bomb suspect 'did not act alone', BBC News
