Wikipedia:Reference desk/Science: Difference between revisions
| (One intermediate revision by the same user not shown) | |||
| Line 1,204: | Line 1,204: | ||
(NOTE: copyrights suspended for this transmission with Wikipedia.) [[Special:Contributions/189.173.210.245|189.173.210.245]] ([[User talk:189.173.210.245|talk]]) 23:25, 18 February 2011 (UTC) |
(NOTE: copyrights suspended for this transmission with Wikipedia.) [[Special:Contributions/189.173.210.245|189.173.210.245]] ([[User talk:189.173.210.245|talk]]) 23:25, 18 February 2011 (UTC) |
||
:Your question has no meaningful physics-based answer, because "inter-dimensional portal" and "new space-time continuum" do not have well-formed, meaningful, physics-based interpretations. Sorry. [[User:Nimur|Nimur]] ([[User talk:Nimur|talk]]) 23:29, 18 February 2011 (UTC) |
:<small>Your question has no meaningful physics-based answer, because "inter-dimensional portal" and "new space-time continuum" do not have well-formed, meaningful, physics-based interpretations. Sorry. [[User:Nimur|Nimur]] ([[User talk:Nimur|talk]]) 23:29, 18 February 2011 (UTC) |
||
:Mind me asking who is that late Dr Hawking? [[Stephen Hawking]] is still alive. [[User:Dauto|Dauto]] ([[User talk:Dauto|talk]]) 02:29, 19 February 2011 (UTC) |
:Mind me asking who is that late Dr Hawking? [[Stephen Hawking]] is still alive. [[User:Dauto|Dauto]] ([[User talk:Dauto|talk]]) 02:29, 19 February 2011 (UTC) |
||
::And Stephen Hawking is normally referred to as Prof. Hawking, not Dr. Hawking. --[[User:Tango|Tango]] ([[User talk:Tango|talk]]) 18:21, 19 February 2011 (UTC) |
::And Stephen Hawking is normally referred to as Prof. Hawking, not Dr. Hawking. --[[User:Tango|Tango]] ([[User talk:Tango|talk]]) 18:21, 19 February 2011 (UTC)</small> |
||
:Anyways, any, '''any''', ANY so called "time machine", i.e. a device/portal/spacetime construction/anything which takes you ''backwards'' in time will be destroyed by [[vacuum fluctuations]] within 10<sup>-43</sup> seconds (The Planck-Wheeler time). So no, even if the question had some meaning, it wouldn't work. [[User:Manishearth|<font color="orange">Manish</font><font color="green">''Earth''</font>]]<sup>[[User talk:Manishearth|<font color="orange">Talk</font>]] • [[Special:Contributions/Manishearth|<font color="green">Stalk</font>]]</sup> 10:22, 19 February 2011 (UTC) |
:Anyways, any, '''any''', ANY so called "time machine", i.e. a device/portal/spacetime construction/anything which takes you ''backwards'' in time will be destroyed by [[vacuum fluctuations]] within 10<sup>-43</sup> seconds (The Planck-Wheeler time). So no, even if the question had some meaning, it wouldn't work. [[User:Manishearth|<font color="orange">Manish</font><font color="green">''Earth''</font>]]<sup>[[User talk:Manishearth|<font color="orange">Talk</font>]] • [[Special:Contributions/Manishearth|<font color="green">Stalk</font>]]</sup> 10:22, 19 February 2011 (UTC) |
||
Revision as of 22:08, 19 February 2011
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
February 11
Depth of soil
Why in somewhere like England, is the depth of soil so great even on the sides or tops of hills? 92.29.123.158 (talk) 00:48, 11 February 2011 (UTC)
- We have an article on the Geology of England which might be a good place to start. Vespine (talk) 01:13, 11 February 2011 (UTC)
- ... and, of course, the soil is extremely thin or even non-existent on the tops of some hills, especially in the Lake District, but peat explains the depth on many hilltops. Dbfirs 07:51, 11 February 2011 (UTC)
- As the geology article mentions, it's partly to do with glaciers, but also why would you expect there not to be soil there? If plants can grow somewhere, which is the case in all of the UK thanks to its relatively warm and damp climate, then eventually soil will begin to build up, even on bare rock. Primary succession and ecological succession aren't particuarly good articles, but do discuss this. SmartSE (talk) 10:28, 11 February 2011 (UTC)
- ... and, of course, the soil is extremely thin or even non-existent on the tops of some hills, especially in the Lake District, but peat explains the depth on many hilltops. Dbfirs 07:51, 11 February 2011 (UTC)
When was astigmatism first detected and corrected by spectacle lenses
I have invented a new type of spectacle frame and patented it, my reason was to enable spectacle lenses to be fitted which include astigmatism for easy construction in developing countrys. Currently best spherical correction is being used by NGOs globaly but its not providing a 100% accurate solution and 10% of the worlds population cannot work because they are refractivly blind. I will be envolved with a TV program made by the BBC shortly and I need to establish who and when astigmatism was first corrected in spectacle lenses. Any help would be appreciated. John D Snelgrove FBDO 86.167.248.127 (talk) 02:42, 11 February 2011 (UTC)
- This book seems to indicate that astigmatism in the eye was independently discovered by Thomas Young in 1800 and later by George Biddell Airy in 1825. Airy is the one who coined the term, as suggested by William Whewell (who also, incidentally, coined the term "scientist"). The first lens that corrected astigmatism was developed in 1824 at Airy's request. As far as pat histories go, this one seems plausible enough to me. Unfortunately you cannot view the citations on Google Books so it is hard to know what the author is basing it on. --Mr.98 (talk) 03:54, 11 February 2011 (UTC)
- In the Bakerian lecture of Young he describes his experiments on his own astigmatism. --Stone (talk) 07:30, 11 February 2011 (UTC)
The Sun during solar maximum
What does the Sun look like through a (properly filtered) 13-cm telescope during solar maximum? I became interested in astronomy in my early teens (and bought a 13-cm telescope), when the Sun wasn't terribly exciting to look at because it was near a solar minimum. I'm much older now, but the Sun is STILL in the same minimum, so I'm very curious about what it would look like when it finally becomes active. --140.180.1.16 (talk) 08:40, 11 February 2011 (UTC)
- First, let me slap on the usual DANGER! DO NOT OBSERVE THE SUN THROUGH A TELESCOPE! warning. Now, if you are "doing it right," you should never even look through a filtered telescope either. You should set your telescope up as a heliostat and project the image of the sun onto an imaging plane - do not use your eye as the focal plane! This is significantly less risky than just using a filter, and produces better images.
- Usually, during solar maximum, the only thing you'll notice is "more sunspots;" but if you're talented at astrophotography, and heavily invest in specific filters, you can observe the chromosphere and the corona. If you're lucky, you may even catch a coronal mass ejection. CMEs can occur at any time, but are much more likely during solar max. To photograph a CME will be difficult. Historically, scientists waited for a total solar eclipse before attempting to photograph the corona; but modern optical equipment and film emulsions can make it possible to attempt chromosphere and corona photography directly, using a completely opaque sunshade. Here are some photos from NASA: Coronal Mass Ejections... (the photos came from the High Altitude Observatory at Mauna Loa Solar Observatory. Never fear; not everyone has access to such equipment. Here's a good "tutorial" that used a 4-inch refractor and a fairly "amateur" 35mm film camera: Observing and Photographing the Solar Chromosphere Through a Solar Cycle. That paper is a little bit old, so they describe using film (instead of digital) cameras; even with filtering, I'd be very nervous connecting my digital camera up to a heliostat, for fear of permanently burning/damaging my sensor. If you know your optics are safely filtered, and have a way to meter the light intensity, you could use a digital camera too. (Do not use your eyes to test your telescope's filter! Permanent eye injury/damage is a serious risk). Nimur (talk) 11:36, 11 February 2011 (UTC)
- A solar filter, which often costs about $50+ USD (full-aperture, NOT eyepiece type, these can crack!!) is usually adequate for viewing the Sun safely by direct optical means, though a small hole or tear could completely compromise safety. A 13-cm telescope is large enough for the Sun's focused rays to do serious damage to the primary and secondary mirrors (assuming reflecting telescope) when using the projection method, and pointing it at the sun is dangerous as well for the finderscope if left uncovered. It is much safer to use both a solar filter and a brightness-reducing eyepiece filter in combination to view the Sun or otherwise leave the outer dust cover on if your telescope has both an outer ring and inner circle objective cover and then apply either projection or aperture-filtered observation, but test it using your camera first to check for any overexposure, although a specially-designed solar telescope is often specifically suited for solar observation but can be far more expensive than the combination of a usual telescope and a solar filter. ~AH1(TCU) 22:08, 11 February 2011 (UTC)
- Thanks for the info. One correction: the projection method definitely doesn't produce better images. There's a reason that people buy H-alpha filters even though they cost thousands of dollars. Even with a simple attenuation filter, you get much higher contrast than by projecting onto a piece of paper. --140.180.0.75 (talk) 01:48, 12 February 2011 (UTC)
- By "better", I meant "bigger image." (Here's an example setup with a 3 foot projection from Sky & Telescope). The sun is huge and bright - so it sustains ridiculous magnification factors; and even with a small telescope, you should have enough resolution to capture a lot of detail when you project up to a 1-foot wide solar image. Now, you'd lose that resolution if your camera sensor were directly in the focal plane unless you've got a full-frame imager and tightly packed pixels and you have the optics to precisely control the image projection on your sensor. Depending on the effective field of view of your camera sensor, and the size of your primary, you probably aren't capitalizing on all available optical resolution of your telescope. "Best" results will of course depend on your intent and your available equipment. Nimur (talk) 18:15, 14 February 2011 (UTC)
Effect of damaging the Earth's core
Not long ago, some classmates presented a plan to dig a tunnel through the Earth's core to provide a high speed train route between New York City and Tokyo. They were unable to answer the question of whether this tunnel would affect the planet's structural stability. Would a tunnel through the core increase the damage that asteroid impacts, etc., could cause? Also, if terrorists managed to detonate a suitcase nuke within the core, what size of earthquake could they cause? (I ask because in 2009, South Korea reported that a North Korean underground nuclear test had created a magnitude 9 earthquake.) NeonMerlin 10:34, 11 February 2011 (UTC)
- There is no conceivable way that a train-sized tunnel would affect the "structural stability" of Earth. What you would need to worry about is whether the tunnel could be structurally sound. But analyzing the hypothetical tunnel's stability is moot - there is no current technology that could be used to dig such a tunnel. Even if the tunnel were not "direct", but instead followed the contour of the earth's surface, we do not have a way to safely excavate a "subway" tunnel underneath the depths of the open ocean across the Pacific. Finally, even if a nuclear weapon could be detonated at great depth (say, even 100 km below the surface, using a hypothetical tunnel or any other proposed mechanism) it is unlikely that it would trigger a catastrophic earthquake. Scientists have been measuring the seismic behavior of nuclear weapons since 1945; the media often grossly misrepresents the energy scales involved. A group at CISAC produced this public report, Technical Analysis of the DPRK Nuclear Test. You may want to re-check where you heard your "magnitude 9" claim; I'm pretty sure that is an inflated number; but it's really irrelevant anyway, because there are so many technical details involved in converting measurements of a bomb into an "equivalent-sized earthquake." The energy released by even the most powerful nuclear bombs is dwarfed in magnitude by even a small earthquake. Nimur (talk) 11:51, 11 February 2011 (UTC)
- (EC) The structural damage is probably negligible - a tunnel is tiny compared to the size of the earth (or the core). For the same reason, a nuclear explosion in the core would not or hardly be noticeable up here. Aside from the engineering difficulties of protecting the tunnel from the stresses and temperatures prevalent in the core, are you aware of the kind of slope the train has to go down and up again when it is near the surface? I'm too lazy to calculate it but it'll probably be more than 45 degrees, much more than any normal train can handle. --Wrongfilter (talk) 11:54, 11 February 2011 (UTC)
- 49 degrees, according to my calculations assuming a spherical
cowEarth. A straight-line tunnel would reach a depth of about 2200 km, penetrating far into the lower mantle, but missing the core by some 500 km. –Henning Makholm (talk) 13:06, 11 February 2011 (UTC)
- 49 degrees, according to my calculations assuming a spherical
- And, as we all know (I assume ;-), a spherical train in a frictionless vacuum moving under the influence of gravity only would need 49 (IIRC) minutes for the trip, regardless of the distance covered. --Stephan Schulz (talk) 14:30, 11 February 2011 (UTC)
- Uniform density and non-rotating Earth are, of course, part of the spherical cow universe! --Stephan Schulz (talk) 19:55, 11 February 2011 (UTC)
- There's one, erm, small technical obstacle to this plan. The inner core rotates relative to the Earth's surface, something like once every 400 years if I remember correctly. As for nukes, they can be detected on a seismograph, but they are very small. Unfortunately Comprehensive Nuclear-Test-Ban Treaty Organization doesn't get into the specifics of the amplitude, but it's something like 3. There's a formula for the size of the bubbles of vaporized rock from a nuke at Underground nuclear testing - but it's something like hundreds of feet near the surface, and in the core I bet the pressure would reduce the size further. Wnt (talk) 18:07, 11 February 2011 (UTC)
- Something else to mention is that plate tectonics all occurs in the Earth's crust, so an explosion far below the crust is unlikely to have much effect. I would suspect that the right-sized nuke, placed at the hypocenter (focus) of the potential earthquake, a few miles underground, typically, could trigger a quake. However, this would only work if strain had built to the point where a quake would occur soon, anyway, and the quake would be no bigger than the normal range for that fault (it might actually be smaller, if it occurs somewhat earlier, with less strain accumulated). Consider an analogy with cloud-seeding to make rain. It doesn't work when there's no moisture in the air, it has to be almost ready to rain anyway. StuRat (talk) 01:20, 12 February 2011 (UTC)
- Actually, the base of the lithosphere (and all plates) is in the uppermost mantle, the asthenosphere, but you're right that you can't cause an earthquake in that way, only trigger it if it's already near a critical point in the earthquake cycle. There are often foreshocks before an earthquake, but opinion is divided as to whether they actually trigger the main event or are just a manifestation of the increasing stress levels in the area where the mainshock happens. Current thinking is leaning towards the latter explanation suggesting that it's actually difficult to trigger an earthquake before it's ready to go. Mikenorton (talk) 10:13, 12 February 2011 (UTC)
Castrating cats
Is it right to castrate domestic cats to make them stay at home? — Preceding unsigned comment added by Sina-chemo (talk • contribs) 11:57, 11 February 2011 (UTC)
- The reference desk doesn't give moral judgements; it only answers factual questions. The article Neutering has information on the positive and negative effects of neutering a cat (but please consult a vet if you have specific questions as we can't give veterinary advice). --Colapeninsula (talk) 12:31, 11 February 2011 (UTC)
- It helps keep the cat from getting testy. Googlemeister (talk) 14:19, 11 February 2011 (UTC)
- No kitten; it really works. Matt Deres (talk) 14:52, 11 February 2011 (UTC)
- Purrr-fect answers. 10draftsdeep (talk) 16:05, 11 February 2011 (UTC)
- I get the feline you're treating this like some kind of joke... Vimescarrot (talk) 18:01, 11 February 2011 (UTC)
- Please, don't make catty comments on the ref desk. It pusses me off. Tinfoilcat (talk) 19:07, 11 February 2011 (UTC)
- Surely a better reason to castrate cats is to avoid getting too many more cats. HiLo48 (talk) 21:46, 11 February 2011 (UTC)
- Uncastrated toms are rather objectionable, doing things like spraying their "scent" on the drapes. If I had such a cat, the only choice would be between castration and putting it to sleep. If it could make the choice, I think it would prefer to skip the nuts. StuRat (talk) 01:03, 12 February 2011 (UTC)
- It is a good idea to castrate them. It doesn't make them stay at home, but it makes them roam, fight and mate less. And, of course, they will not produce unwanted offspring. You should do this to your cats unless you are breeding them. Zzubnik (talk) 14:57, 14 February 2011 (UTC)
- Castration would seem an in effective method of birth control. Unless every single tom in a neighborhood is castrated, a queen in heat will find a willing an capable mate. Spay (ovario-hysterectomy) 50% of the stray queens and you cut the birth rate in half. Castrate 95% of the toms and you have no effect at all. -- 119.31.126.67 (talk) 00:34, 16 February 2011 (UTC)
differentiation by first principles
how to obtain derivative of e to the power root x by using limits? —Preceding unsigned comment added by 180.215.34.14 (talk) 12:28, 11 February 2011 (UTC)
- This is a duplicate of a recent mathdesk question, probably by the same anonymous user: WP:RD/MA#differentiation by first principles. Please don't ask the same question on several desks. –Henning Makholm (talk) 12:44, 11 February 2011 (UTC)
- If you read closely, you'll see that this is actually a different question, in that it adds the specification "...by using limits". The answer given on the math ref desk used various rules about differentials, rather than using just the definition of the derivative as a limit, and expressing a derivation of the result by using expressions involving limits. Red Act (talk) 22:40, 11 February 2011 (UTC)
Please solve USING LIMITS. —Preceding unsigned comment added by 180.215.44.196 (talk) 07:55, 12 February 2011 (UTC)
- It follows by definition if you choose characterization 4 from Characterizations of the exponential function! Anyway the maths reference desk is the right place. Dmcq (talk) 12:13, 12 February 2011 (UTC)
Extra 4 minutes
If a day is 23 hours and 56 minutes long instead of exactly 24 hours, how do the extra 4 minutes fit in? jc iindyysgvxc (my contributions) 12:52, 11 February 2011 (UTC)
- It's the difference between the solar day and the sidereal day. The Earth rotates once every 23 hours and 56 minutes with respect to the stars. But it also circles the sun (or, geometrically equivalently, the sun circles the Earth) once per year. So the Earth has to do 4 minutes worth of catching up to the relative movement of the sun every day. Note that 365*4 minutes is nearly exactly one day, i.e. the extra rotation due to the motion around the sun. --Stephan Schulz (talk) 13:00, 11 February 2011 (UTC)
- In other words, if we measure the day by tracking the location of the sun, we would have to wait an extra 4 minutes before we turn a full 360 degrees. And that is the source of the discrepancy. That's because the earth has moved along a small distance in its orbit of the sun between today and yesterday. --Jayron32 13:46, 11 February 2011 (UTC)
- Thanks. I've heard the term "sidereal" and read of the difference but never had it explained so clearly in terms of "one day per year." Edison (talk)
- Supposing for the sake of simplicity that the year was exactly 365 days long, the sidereal day would be exactly 365/366 of the solar day, and the number quoted above as "4 minutes" would turn out to be be exactly 1/366 of a year. --Anonymous, 03:40 UTC, February 12, 2011.
- Thanks. I've heard the term "sidereal" and read of the difference but never had it explained so clearly in terms of "one day per year." Edison (talk)
- In other words, if we measure the day by tracking the location of the sun, we would have to wait an extra 4 minutes before we turn a full 360 degrees. And that is the source of the discrepancy. That's because the earth has moved along a small distance in its orbit of the sun between today and yesterday. --Jayron32 13:46, 11 February 2011 (UTC)
Leap years
then why do we leap 1 day every 4 years? —Preceding unsigned comment added by 165.212.189.187 (talk) 16:52, 11 February 2011 (UTC)
- That's another matter entirely. We leap a day every so often because a year is actually slightly longer than 365 days. Dauto (talk) 17:05, 11 February 2011 (UTC)
- In other words, it takes about 365 1/4 turns on the earth's axis to arive at the same point in the earth's orbit. That extra 1/4th of a day adds up and needs to get tacked on every 4 years. --Jayron32 18:09, 11 February 2011 (UTC)
- Specifically, 97 times every 400 years because it takes 365.24 turns. :) —Preceding unsigned comment added by 205.193.96.10 (talk) 20:45, 11 February 2011 (UTC)
- The "one day per year" is because 364 days are due to the Earth turning, and the last one is because we are going around the sun. If the Earth didn't rotate at all, we would still get one day per year due to us going around the sun. Leap days, on the other hand are caused because it takes an extra 0.24 days to get around the sun and start the seasons over; it has nothing to to with the Earth's own rotation. —Preceding unsigned comment added by 205.193.96.10 (talk) 20:49, 11 February 2011 (UTC)
- In other words, it takes about 365 1/4 turns on the earth's axis to arive at the same point in the earth's orbit. That extra 1/4th of a day adds up and needs to get tacked on every 4 years. --Jayron32 18:09, 11 February 2011 (UTC)
- See leap year and leap second. The sideral motion of the Moon also varies in this matter as it catches up to the Earth on one side of its orbit while remaining gravitationally locked to face one side to Earth. ~AH1(TCU) 21:59, 11 February 2011 (UTC)
Radio power
Hi, does anyone know how much radio signal power is needed in order to pick up a station on an ordinary household radio (with no special aerial or other fancy equipment)? I'm talking about the power "in the ether" at the place where the radio is located, not the total power of the transmitter, which is at an unspecified distance. I think I'm looking for an answer in watts/m^2 (if not, please correct me). Does the answer differ much between FM and AM? 86.179.4.118 (talk) 14:39, 11 February 2011 (UTC)
- The integrated power at the aerial terminal is given (albeit without source) in Orders of magnitude (power) as on the order of femtowatts for an FM signal and a good-quality radio receiver, but I'm not sure what the appropriate 'effective area' would be for the aerial. A similar figure (roughly 10 fW) is given for a minimum receivable spread-spectrum cellular telephone signal. As a very rough (order of magnitude) estimate, a detectable FM signal flux is going to be on the order of femtowatts to picowatts per square meter, depending on the size and configuration of your antenna. TenOfAllTrades(talk) 15:24, 11 February 2011 (UTC)
- See "Field strength." The "signal power" of a broadcast signal at the receiver location is typically given in units of microvolts per meter, rather than in watts per square meter. Then the signal at the radio's antenna terminal or first RF input stage is affected by the type and orientation of the antenna, or the "gain" of the antenna. Modern radios often use a little ferrite loopstick in the radio, while older ones used a flat coil on the back panel, and older ones still, with less RF amplification, required a long overhead antenna and an Earth ground. Naturally a weaker signal or stronger signal will produce an audio output with varying signal to noise ratio, and with varying clarity compared to atmospheric static, interference from electrical equipment, thermal noise in the receiver circuitry and interference from other stations on the same or adjacent frequencies. The bandwidth affects the signal to noise ratio. A signal to noise ratio of 3 deciBels is sometimes described as the minumum usable signal, but it would sound horrible. A 10 dB SNR is often used in describing the sensitivity of a receiver, per [1]. Someone with recent skills or training in communications calculations might be able to come up with the microvolts per meter for a 10 dB Signal plus noise to noise ratio on a typical (say AM) receiver in the broadcast band. I once saw a map of Italy showing the signal strength at varying locations in that country, regardless of what the transmitter was, but I haven't seen that for the US. Says that the sensitivity of modern radio receivers ranges from several microvolts to several millivolts. I suppose that to convert this to "power" you would need to know the input impedance of the radio at the antenna connection, which for most ordinary AM radios is somewhere internally where the loopstick connects to the first RF stage. Edison (talk) 16:00, 11 February 2011 (UTC)
- You can approximately convert electric field strength (volts/meter) to radiant intensity (watts per square meter) by squaring and dividing by the antenna impedance; in practice, you need to account for the antenna matching properties, impedance of free air, antenna directivity, and so on. A comprehensive theoretical treatment and several useful conversion tables are provided by Antarctic Impulse Transient Antenna website at University of Hawaii: Field Intensity and Power Density, which is an excerpt from the Navy Electronic Warfare & RADAR Systems Handbook. Nimur (talk) 22:14, 11 February 2011 (UTC)
James Watt steam engine patent
I am looking for the patent that James Watt filed in 1784. This patent is mentioned in [article "History of Rail Transport"], but I cannot locate an electronic copy of the patent itself. Thanks. 128.223.222.16 (talk) 16:30, 11 February 2011 (UTC)
- I don't see it cited there or at James Watt#Early experiments with steam or even anything specifically about the 1780s at all in Watt steam engine. That's bad. DMacks (talk) 19:25, 11 February 2011 (UTC)
- I looked briefly at some old Google Books entries about Watt's 1784 patent; it seems to be a real thing, but none of them gave patent numbers. The European patent office search doesn't seem to let you search very old patents very easily, even though they may be on there (like the 1769 Watt patent linked in our article). Seems like it is not going to be a very easy thing to dig up online. --Mr.98 (talk) 21:54, 11 February 2011 (UTC)
Your local library may be the only source at the moment. According to Birmingham City Council the European Patent Office is currently embarked on the "digitisation of UK Patents 1870 - 1920", although this espacenet publication contradicts that and suggests that there's not much hope for anything before 1890.
- Got it in three ! Project Gutenberg has Kinematics of Mechanisms from the Time of Watt by Eugene S. Ferguson which has a very detailed history of Watt's production, competition, and patents, including a diagram and description of "Watt's mechanisms for guiding the upper end of the piston rod of a double-acting engine (British Patent 1432, April 28, 1784)". SamuelRiv (talk) 20:19, 12 February 2011 (UTC)
Bengal fire
What exactly is Bengal fire (a.k.a. Bengal light), and how did it get its name? All I found on the web so far is this – and many indirect uses, such as flowers and tea carrying that name. — Sebastian 21:21, 11 February 2011 (UTC)
- This Everything2 post explains that it is a firework, mostly potassium nitrate, adulterated with copper and/or barium to add a blue-green color. Is is so named because these fireworks/explosives were originally originally manufactured and supplied from Bengal to England. The Everything2 node isn't quite a reliable source; and though it cites a few references, they are also of dubious quality. But this journal article, Pyrotechnics in Fireworks (2004), seems to corroborate the general claims, though. It seems that other "brightly colored" things use the term "bengal fire" because it sounds neat. Nimur (talk) 21:41, 11 February 2011 (UTC)
- Thank you, Nimur! — Sebastian 22:20, 11 February 2011 (UTC)
- Actually, as I'm trying to incorporate that information, I noticed that we used to have a redirect from Bengal fire to Bengal fire stick. I restored the latter in my user space so I can ask here if there's anything to it; it is at least not an obviously fictitious page, as the deleters assumed. — Sebastian 22:45, 11 February 2011 (UTC)
- I recall purchasing "Bengal matches" around November 5th many years ago. Were they an early alternative to sparklers? Dbfirs 00:20, 12 February 2011 (UTC)
February 12
Boa constrictor scientific naming...
According to the Boa constrictor article - "Though all boids are constrictors, only this species is properly referred to as "Boa constrictor"; an almost unique instance of an animal having the same common and scientific binomial name". So, which came first - the common name, or the scientific name? --Kurt Shaped Box (talk) 04:15, 12 February 2011 (UTC)
- I take that sentence to mean that it has always been known in English as a boa constrictor. Since that is its common name and its scientific name how can one say that either came first? (Of course its South American name jibóia was presumably used before that.)--Shantavira|feed me 07:57, 12 February 2011 (UTC)
- Linnaeus created this name in 1788, and he wrote in Latin, so if we equate "scientific binomial name" and "Latin name given by Linnaeus" then it would appear that the scientific name came first. The Penny Cyclopedia in 1836 referred to the name as "popular", so the transition from scientific to popular came between those two dates. The Latin word boa, incidentally, goes all the way back to Pliny. [Source: OED] --Heron (talk) 11:23, 12 February 2011 (UTC)
In some cases, it would be redundant, as in Gorilla gorilla! --FOo (talk) 18:13, 13 February 2011 (UTC)
when the earth was shining
the first earth surface might be very hot between 2000~3000 degrees centigrade . this temperature cases the rocks be melt then this hot cases the matter such as the volcanic magma to shine . before the cooling surface of earth and when the sun was young it is clear that the earth was red color and shining . how much years was the during of this condition . (all remarks are collected from astronomic articles)--78.38.28.3 (talk) 04:54, 12 February 2011 (UTC)a. mohammad zade Iran 2011
- We don't know very much about the Hadean period, but our article History of the Earth might be of interest. If the Earth initially shone, then it could have done this for only a comparatively short time, perhaps between 4,540,000,000 and 4,530,000,000 years ago (when the moon was formed and the Earth had a crust). Dbfirs 10:33, 12 February 2011 (UTC)
- Use of the word "shine" in this context is potentially confusing, since it is frequently used in astronomy, as elsewhere, to denote the reflection of incident light, as in Earthshine. Colloquially, all the planets (and their satellites), including the Earth, "shine" by reflecting the light of the Sun (Earthshine being a double reflection), according to their individual albedos. For the phenomenon you are interested in, incandescence, or colloquially "glow", would be more appropriate. 87.81.230.195 (talk) 13:14, 12 February 2011 (UTC)
- Which incident light does the Sun reflect to create sunshine, then? –Henning Makholm (talk) 15:22, 12 February 2011 (UTC)
- I said "potentially confusing", "frequently" and "more appropriate", not "wrong", "invariably" or "exclusively," and "solely appropriate". I was also pointing the OP towards articles on subjects relevant to his query, and providing hopefully polite guidance on vocabulary usages in a language in which he is evidently not fluent. How helpful do you suppose your comments were? *Stalks off in high dudgeon.* 87.81.230.195 (talk) 22:50, 12 February 2011 (UTC)
Yes, your reply was perfectly acceptable and you provided useful links, and we weren't really complaining about it, but we perfectly understood the usage of the OP whose first language is not English. Apologies for any offence caused
. Dbfirs 08:37, 15 February 2011 (UTC)
only one of my friends understood my meaning . I know that the earth is reflecting the sun shine all over the life of that and all matters such as dark matter has shining . the last planet that nasa discovers is melt and hot and is shining
so it can shine without reflecting the other star light--78.38.28.3 (talk) 04:51, 13 February 2011 (UTC) a. mohammadzade]] —Preceding unsigned comment added by 78.38.28.3 (talk) 04:46, 13 February 2011 (UTC)
Please see the articles on the Hadean period, Giant impact hypothesis, Cool Early Earth, and Late Heavy Bombardment. Because rocks from asteroids date back to 4.6 billion years, and rocks from Earth date back only to 3.8 billion years, it was thought the surface was largely molten for 800 million years, but now some people think that it was solid for most of this time, until a wave of asteroid bombardment. But according to Giant impact hypothesis, the existence of a magma ocean has never actually been proved at all! Unfortunately, the answer here is that it just isn't known yet. Wnt (talk) 07:55, 13 February 2011 (UTC)
Borosilicate glass
r light bulbs Borosilicate glass — Preceding unsigned comment added by Tomjohnson357 (talk • contribs) 05:15, 12 February 2011 (UTC)
- If you're talking about ordinary incandescent lamps, then the outer envelope can be soft soda glass, which is cheaper to make and form. However, the inner glass stem that the metal supports are fixed to, are of borosilicate due to its low coefficient of expansion. Other lamps that run very hot, like vapour discharge lamps, are mostly borosilicate glass throughout.--Aspro (talk) 10:55, 12 February 2011 (UTC)
capacitance
4 μF capacitor is charged by 250V.Find energy stored in it.It is connected in parallel combination to an uncharged 3μF capacitor. Find energy stored. —Preceding unsigned comment added by 180.215.44.196 (talk) 07:41, 12 February 2011 (UTC)
- Welcome to the Wikipedia Reference Desk. Your question appears to be a homework question. I apologize if this is a misinterpretation, but it is our policy here not to do people's homework for them, but to merely aid them in doing it themselves. Letting someone else do your homework does not help you learn nearly as much as doing it yourself. Please attempt to solve the problem or answer the question yourself first. If you need help with a specific part of your homework, feel free to tell us where you are stuck and ask for help. If you need help grasping the concept of a problem, by all means let us know.
- Have you read our Farad article? It gives various equations that relate the charge, voltage, and stored energy to the capacitance. For the first question, use the appropriate equation directly. For the second question, the first capacitor will discharge (dropping its voltage) into the second until the voltage on both is the same; no charge is lost. Find the initial charge on the first capacitor, then find the corresponding volatage on the combined system. CS Miller (talk) 10:20, 12 February 2011 (UTC)
Radio activity
A radio active material at a given instant emit 3000 particles per minute,10 minute later it emits 1500 particles per minute. find decay constant and half life. —Preceding unsigned comment added by 180.215.44.196 (talk) 07:45, 12 February 2011 (UTC)
- We avoid homework questions as if they were radioactive. Clarityfiend (talk) 07:55, 12 February 2011 (UTC)
- I suggest you read decay constant and half life.--Shantavira|feed me 08:36, 12 February 2011 (UTC)
Legal chemistry
Is there and article regarding legal chemistry?--Email4mobile (talk) 10:35, 12 February 2011 (UTC)
- Probably forensic chemistry. Someguy1221 (talk) 10:39, 12 February 2011 (UTC)
- Or forensic toxicology, as the original use of the term 'legal chemistry' was all about poisoning. Mikenorton (talk) 10:43, 12 February 2011 (UTC)
- Thanks a lot. I will add Arabic interwiki to that articles.--Email4mobile (talk) 11:01, 12 February 2011 (UTC)
Changing the length of a second/minute/hour/day/year
The recent leap day related questions left me with a question that I don't think has been addressed. Has the idea of changing the official length of a day (or whatever would be necessary) so that we don't need as many/any leap days been seriously proposed? Or are we at an optimum now? Dismas|(talk) 10:44, 12 February 2011 (UTC)
- Well, if you change the length of the day, so that the year has exactly 365 days, then noon will be shifted from 12:00 to almost 18:00 within a year - I don't think that's acceptable. Icek (talk) 11:11, 12 February 2011 (UTC)
- Exactly. The fundamental problem is that the length of the tropical year – essentially, the length of one full cycle of seasons – just isn't an integer multiple of the solar day. If we insist that our timekeeping system stays in sync with the sun (12:00 noon is always in the middle of the daytime) and with the seasons (the winter solstice will always fall in late December), then these little corrections are unavoidable. TenOfAllTrades(talk) 15:35, 12 February 2011 (UTC)
- Proposal to abolish leap seconds have been put forward by people who don't understand the issue properly. I think the current arrangement will survive for sometime yet and is as good as is reasonable and workable. --Aspro (talk) 11:12, 12 February 2011 (UTC)
- The second is an arbitrary human unit, unlike the solar day and tropical year, though in practice it's too entrenched to be redefined. As I software engineer I don't like the current system of random leap seconds announced six months in advance. It's akin to announcing leap years on March 1 of the previous year. I'd much prefer a predefined schedule. The schedule doesn't have to be "no leap seconds ever", it just has to be predictable. -- BenRG (talk) 18:18, 12 February 2011 (UTC)
- That's why things are as good as can be expected . The change in the Earth's rotation can't be modelled very well yet based on our current understanding. Even changing the way a second is defined will not get around this fact. To expect more at present is just wishful thinking. --Aspro (talk) 18:47, 12 February 2011 (UTC)
- The problem is that the need for leap seconds is inherently unpredictable; the rotation rate of the Earth just isn't regular enough for us to predict the need for a leap second well in advance. Between 1988 and 1998, there were eight leap seconds added; between 1998 and 2008, there was just one: File:Leapsecond.ut1-utc.svg. In principle, if one were willing to tolerate an increased drift in UTC versus mean solar time, one could implement a longer period between leap second adjustments or allow a larger deviation to accumulate before corrections were applied — but it still wouldn't prevent those adjustments from being at 'random' intervals. TenOfAllTrades(talk) 19:00, 12 February 2011 (UTC)
- The second is an arbitrary human unit, unlike the solar day and tropical year, though in practice it's too entrenched to be redefined. As I software engineer I don't like the current system of random leap seconds announced six months in advance. It's akin to announcing leap years on March 1 of the previous year. I'd much prefer a predefined schedule. The schedule doesn't have to be "no leap seconds ever", it just has to be predictable. -- BenRG (talk) 18:18, 12 February 2011 (UTC)
- A perfectly workable solution would be simply to abolish leap seconds and let UTC float with respect to the Earth's rotation. The offsets between local mean solar time and civil time that we already accept as unproblematic everyday consequences of zonal time are far larger than the UTC drift that could accumulate until several centuries pass. And once those centuries do pass, countries can simply choose to switch to another UTC offset for their civil timekeeping. Many countries implement such switches twice per year already, and modify their rules for these changes relatively frequently. Simply skipping one of the diurnal switches in order to move one timezone east or west will be a fairly routine exercise, compared to the practical benefits a predictable "universal time" scale would bring.
- Alternatively we could start defining civil time everywhere in terms of hourly offsets from TAI and abolish the entire concept of a separate UTC scale. This would be just as workable in the long term, but the half minute of TAI-UTC difference that has already accumulated would make the switchover impossibly chaotic as a practical matter, and for no real benefit compared to a floating UTC. –Henning Makholm (talk) 02:27, 13 February 2011 (UTC)
- Floating universal time (GMT) was abandoned because it became impractical and got replaced by UTC. Satellite navigation may remove some of the current dependence on this, but one still has the problem of how to constantly synchronise all the independent time pieces at odd times throughout the year which may actually require more disruption than the present system. Transaction in commerce are completed and logged in milliseconds. Television transmission networks which are slightly out of sync falter. However, the suggestion to use ITA with offsets, would not only work, but is what is done now, but to let ITA loose and let it drift away as civil time and away from the astronomical time datum, would make future interpretation of data (especial astronomical data) more difficult. All the pros and cons added together leave us with what we have now, as the best compromise, and so we wont start going backwards on the basis of spurious arguments. --Aspro (talk) 12:38, 13 February 2011 (UTC)
- Um, GMT does not float; on the contrary it is explicitly tied to the Eath's rotation by definition. In a sense, what is done now is to use ITA with offsets, except that those change unpredictably, leaving gaps and overlaps in the otherwise continuous time scale. Sure, astronomers need to keep accurate track of the Earth's rotation for their own particular reasons -- they shall be welcome to keep historical records about that so they can interpret their observations in the future -- but that is not a good reason to shackle all of the rest of humanity to an unpredictable, non-continuous, non-arithmetic civil timekeeping scale. –Henning Makholm (talk) 16:34, 13 February 2011 (UTC)
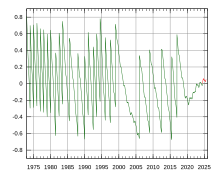
- You say: Um, GMT does not float; on the contrary it is explicitly tied to the Eath's rotation. Please read Non sequitur (logic). See image . I don't mind explaining things but please don't come back with absolute tosh!--Aspro (talk) 18:16, 13 February 2011 (UTC)
- The question is, if you add seconds to the end of every day, where to is circa 1820 and a ≈ 0.0014/day/century, what's the expected maximum drift from solar time over, say, the next thousand years? I don't know the answer, but if it's, say, ten minutes, then I think it would be a huge improvement over what we've got now. I'm pretty sure the number of people who don't need to correct for the current 1-second drift but would need to correct for a ten-minute drift is small compared to the number of people inconvenienced by the current system. For heaven's sake, we tolerate a 0.3% variation in calendar year length and a whopping 10% variation in calendar month length. Astronomers have to correct for those too. -- BenRG (talk) 22:45, 13 February 2011 (UTC)
- The short term fluctuations are random and 1820 was the nadir of a parabola, which means the drift does not follow a liner increase - so that doesn't work. Recalculating every day or other regular period and adjusting, goes against every other method of standardization so that's not on either. --Aspro (talk) 19:55, 14 February 2011 (UTC)
- Not to mention that we tolerate a ±30 minute deviation from solar time just so that our time zones can change on one-hour boundaries rather than one-minute or one-second. Why is one-second accuracy in UTC so important? -- BenRG (talk) 23:07, 13 February 2011 (UTC)
- It is not about looking out the window a noticing anything different. The current time systems have evolved as technology has advanced to benefit from evermore sophisticated time standards. Leap seconds are one of those steps to bridge the gaps until the next advance is ripe for adoption. Abandoning them because some people don't care about the problems it will cause in multiple other places is not the way to go. For computer systems the Network Time Protocol adjusts for leap seconds automatically. Systems for which this approach is unsuitable, can stick with ATI as standard time reference.--Aspro (talk) 19:55, 14 February 2011 (UTC)
- I'm aware of the existence of NTP and TAI. Other than that, I can't make any sense of your reply. Isn't "the next advance" what this crazy subthread is about? -- BenRG (talk) 21:27, 14 February 2011 (UTC)
- Did this current system come about over-night? No, it didn't! From the time when the ancient Egyptians just counted the days and ignored the astronomical year, the advancement of the measurement of time has advanced stepwise. The introduction of leap seconds is just one of those many steps. At each step, some individuals have objected, as it has forced them to adopt new ways of doing things. I don't think there is any point in trying to explain this in simpler terms as you appear to be not trying to understand. A trait that others, who object to leap seconds, seem to share IMHO.And which you appear to be now confirming.--Aspro (talk) 21:54, 14 February 2011 (UTC)
- I'm aware of the existence of NTP and TAI. Other than that, I can't make any sense of your reply. Isn't "the next advance" what this crazy subthread is about? -- BenRG (talk) 21:27, 14 February 2011 (UTC)
- It is not about looking out the window a noticing anything different. The current time systems have evolved as technology has advanced to benefit from evermore sophisticated time standards. Leap seconds are one of those steps to bridge the gaps until the next advance is ripe for adoption. Abandoning them because some people don't care about the problems it will cause in multiple other places is not the way to go. For computer systems the Network Time Protocol adjusts for leap seconds automatically. Systems for which this approach is unsuitable, can stick with ATI as standard time reference.--Aspro (talk) 19:55, 14 February 2011 (UTC)
- The question is, if you add seconds to the end of every day, where to is circa 1820 and a ≈ 0.0014/day/century, what's the expected maximum drift from solar time over, say, the next thousand years? I don't know the answer, but if it's, say, ten minutes, then I think it would be a huge improvement over what we've got now. I'm pretty sure the number of people who don't need to correct for the current 1-second drift but would need to correct for a ten-minute drift is small compared to the number of people inconvenienced by the current system. For heaven's sake, we tolerate a 0.3% variation in calendar year length and a whopping 10% variation in calendar month length. Astronomers have to correct for those too. -- BenRG (talk) 22:45, 13 February 2011 (UTC)
- ±30 minutes is only the minimum necessary deviation. In practice, there are places that use civil timescales as much as 90 minutes from local mean solar, such as Galicia (Spain) (150 minutes in the summer), Iceland or Western Sudan. –Henning Makholm (talk) 00:34, 14 February 2011 (UTC)
Physics Entries in General
I am new to wikipedia. I am writing a book on evolution and my areas of expertise are biology, animal behavior and evolution. I am however writing an introductory chapter on atoms in order to define where life does not appear to exist ( in order to later identify the first molecules which could be considered to have "life"). As I have been reviewing the elementary particles such as quarks and gluons, etc I have found a very disturbing trend to not clearly define right up front which aspects of particles and theories have been clearly "proven" and observed and which concepts are theoretical in nature. I feel that even as not being a physics specialist, that this concept regarding scientific theories is extremely basic. I added some comments to the gluon page in this regard and they were removed. I have no wish to "soapbox" about this concept. But I have noticed for physics based articles, often the pages will immediately start going off on very mathematic based formulas without even stating whether there is any proof of the particle or concept. In biology I can talk about tree bark and vascular systems and photosynthesis and we can go in and very soundly prove and display and observe the science right down to the molecules which house the chemical reactions from sunlight to sugars. But the particle folks go into arcane mathematics which is almost religious in nature without ever clearly stating whether for example particle spin (1/2 or full integer) is theorized or has actually been observed. It is like smoke and mirrors with math being the mystical gate. In other words "regular" people cant consider the accuracy of these theories as they immediately made opaque by the insertion of mysterious terms and equations. I think this concept is very basic and extremely unscientific. How does one taker on these articles in order to demand very basic descriptions of what exactly is proven and what exactly is theoretical up front? Thanks so much for your time. — Preceding unsigned comment added by Maplelanefarm (talk • contribs) 14:47, 12 February 2011 (UTC)
Again..thanks for your time...I think this is a huge issue with most of the particle pages. — Preceding unsigned comment added by Maplelanefarm (talk • contribs) 14:49, 12 February 2011 (UTC)
- Indeed, you do need to know mysterious terms and equations to make sense of the subatomic world. There isn't much of a way around that. There are experiments, say, that show that spin certainly exists. But even interpreting what is going on in the experiment requires you to buy into a lot of complicated maths. Most of the standard model is "proven" (as far as that goes) pretty definitively. The stuff that is wholly "theoretical" lies just on the edges — e.g., quantum gravity, string theory, etc. The presence of math does not indicate that something is not experimentally verified. As just an indication, the classic experiment proving the existence of spin is the Stern–Gerlach experiment. It doesn't rely on heavy maths, but it still requires quite a deep theoretical understanding to make sense of what is going on. I'm inclined to think this is an artifact of the physics, not so much Wikipedia, though I do agree that the high levels of technical jargon in such articles make them pretty impenetrable. --Mr.98 (talk) 15:15, 12 February 2011 (UTC)
- Why do you say you can "prove...and observe the science right down to the molecules..."? The tools you use to do that such as electron microscopes and mass spectrometers all rely on physics concepts like particle spin and electron orbitals. To properly complete your "proof" you will need these concepts too. Franamax (talk) 15:56, 12 February 2011 (UTC)
- There is no royal road to physics. If you really want to understand the subatomic world you will need to learn some math along with it. Said that, I think you do have a point that many of those concepts can be partially explained in plain language but that s not a particularly easy thing to do. Plain language is just inadequate for physics concepts and that is why we use math to begin with. Also, there isn't a bright line separating "known facts" from "theoretical constructs" since the theories are an indispensable component of how physics is understood. Dauto (talk) 16:22, 12 February 2011 (UTC)
- consider the first sentence of gluon : "Gluons are elementary particles ..." vs tachyon "A tachyon is a hypothetical subatomic particle ..." and magnetic monopole "A magnetic monopole is a hypothetical ...". Probably, if the article does not mention "hypothetical" in the first few sentences, you may consider it "proven". I suspeci it is the same in biology, where the articles about real animals will not mention they are real, while articles about mythical animals mention they are mythical. 83.134.173.228 (talk) 16:52, 12 February 2011 (UTC)
- Here is a quote from the lead of quark's article:
- "Quarks were introduced as parts of an ordering scheme for hadrons, and there was little evidence for their physical existence until deep inelastic scattering experiments at SLAC in 1968.[6][7] All six flavors of quark have since been observed in accelerator experiments; the top quark, first observed at Fermilab in 1995, was the last to be discovered.[5]"
- Makes me wonder what are you complaining about?
- Did you even read the lead?
- Dauto (talk) 19:14, 12 February 2011 (UTC)
- It's worth remembering that all serious extensions of the Standard Model (SM) are equally compatible with the experimental facts. There are no known facts that completely rule out supersymmetry or technicolor and yet there is no experimental evidence that we should accept them over the plain old SM. How would you say one is "proven" and the other not? Certainly neither is falsified. 129.234.53.49 (talk) 18:57, 12 February 2011 (UTC)
- All of the standard model particles except for the Higgs boson have been experimentally observed. None of the additional particles required by either supersymmetry or technicolor have been experimentally observed. That's the difference. Dauto (talk) 19:09, 12 February 2011 (UTC)
- Can you explain what you mean by "experimentally observed"? As far as I'm aware, an extension of the SM with appropriately broken SUSY would have the same low-energy scattering signatures as the SM itself. The fact that at some point the two models would lead to different pheneomena doesn't mean that there is any more experimental evidence to support one over the other. 129.234.53.49 (talk) 20:26, 12 February 2011 (UTC)
- I'm not saying SUSY is wrong. But there is no indisputable experimental evidence that it is right either. It is still a hypothesis. For instance, there is no experimental evidence that the selectron actually exists .On the other hand, there is plenty of experimental evidence that the particles of the standard model actually exist. That is a huge difference. Of course that doesn't mean that SUSY is wrong. Dauto (talk) 20:40, 12 February 2011 (UTC)
- Well I'm not trying to defend or refute SUSY, that was just an example. My point is that I don't think there is such a thing as "experimental evidence that [some particles] exist". You don't observe proof that something exists, you see experimental results which agree (or disagree) with your theory. Any reasonable extension of the SM will agree with the data gathered so far just as well as the SM does. They are both hypotheses, which are currently not falsified; all current support for the SM is also support for the MSSM and many other theories too. 129.234.53.49 (talk) 23:02, 12 February 2011 (UTC)
- I'm not saying SUSY is wrong. But there is no indisputable experimental evidence that it is right either. It is still a hypothesis. For instance, there is no experimental evidence that the selectron actually exists .On the other hand, there is plenty of experimental evidence that the particles of the standard model actually exist. That is a huge difference. Of course that doesn't mean that SUSY is wrong. Dauto (talk) 20:40, 12 February 2011 (UTC)
- Can you explain what you mean by "experimentally observed"? As far as I'm aware, an extension of the SM with appropriately broken SUSY would have the same low-energy scattering signatures as the SM itself. The fact that at some point the two models would lead to different pheneomena doesn't mean that there is any more experimental evidence to support one over the other. 129.234.53.49 (talk) 20:26, 12 February 2011 (UTC)
- All of the standard model particles except for the Higgs boson have been experimentally observed. None of the additional particles required by either supersymmetry or technicolor have been experimentally observed. That's the difference. Dauto (talk) 19:09, 12 February 2011 (UTC)
- Yes, I understand what you're saying but I think the point you're making is a bit disingenuous. The SM is consistent with the data gathered so far because we have gazillions of experiments confirming it. SUSY is consistent with the experiment because we have gazillions of experiments that neither confirm nor refute it. It is not the same thing. There is a difference between being confirmed by experiment and being consistent with experiment. Dauto (talk) 06:02, 13 February 2011 (UTC)
- It looks like we may have to agree to differ here. I simply disagree with your last statement; I just can't see how "confirmation" by experiment would work. 129.234.53.49 (talk) 22:35, 13 February 2011 (UTC)
- Yes, I understand what you're saying but I think the point you're making is a bit disingenuous. The SM is consistent with the data gathered so far because we have gazillions of experiments confirming it. SUSY is consistent with the experiment because we have gazillions of experiments that neither confirm nor refute it. It is not the same thing. There is a difference between being confirmed by experiment and being consistent with experiment. Dauto (talk) 06:02, 13 February 2011 (UTC)
- The OP is writing a book, and complaining that Wikipedia's content makes it difficult to evaluate scientific facts. If the original poster is writing a serious book, they should consider hiring a subject-matter expert to review any scientific writing. You may be able to find a local scientist or professor who can contract to review your work for scientific accuracy. You can also contract to various "review shops" on the internet. Physics is difficult; and presenting it correctly and accurately is a challenge. But if any content you wrote were ever challenged, it would be far better to say "...but the content was reviewed for scientific accuracy by Professor So-and-So at Podunk University," rather than "...the content was based on my own interpretation of Wikipedia." A more serious book should be reviewed by somebody with more serious credentials. Physics is an ultra-precise subject, and subtle variations that you may use when wording your presentation of a topic can mean the difference between "completely validated by scientific experiment" and "completely wrong." This applies even to the classical and well-established (non-mind-bending) areas of physics, like Newtonian descriptions of force and energy. "Regular people" can evaluate anything they want for accuracy - but if "regular people" are disinclined from being extremely precise in their thought-process, they will never be able to evaluate physics - no matter how simple and straightforward any presentation may be. Nimur (talk) 20:47, 12 February 2011 (UTC)
- I'm not sure whether I'm on the right track here, but the OP seems particularly concerned about counterintuitive notions like spin. The article there explains that spin does not obey the usual rules we expect spinning objects to, so it isn't the same sort of thing that a spinning beach ball has, but perhaps our articles don't make these things terribly explicit. As I understand it, spin is best seen as a mathematical idea that works in quantum mechanics in a way similar to how orbital momentum works for beach balls. The resemblance could stop there, if it should happen to violate intuition, or it could go further, though I myself do not know any more about it. To say that an electron has spin 1/2 is impossible to picture, but it simply means that when you do the math, you find the electron takes a 720 degree rotation (or something mathematically equivalent) in order to reach a state isomorphic to its original one. To say further that it has been demonstrated experimentally only means that we have so much evidence that we have got the mathematics right that we can teach it at undergraduate level without fear of embarrassment. Someone might come up with some deeper mathematics, or a better verbal description, later, but for now we're doing alright. Can a physicist please tell me if I've got this right, and can the OP perhaps tell me if it helps any? It's been emotional (talk) 16:56, 13 February 2011 (UTC)
- I certainly agree that Wikipedia has an ongoing problem with math and science articles written by experts, for experts, as opposed to the general population. The sticking point in simplifying such articles is always that the simplified model is not quite as correct. For example, all Newtonian physics is technically "wrong", since it doesn't account for time dilation, etc., and other effects of relativity. So, you can't say that a train going 10 km/hr will travel 10 km in an hour, if any physics PhDs are around, as it all depends on the locations of the observers, etc. StuRat (talk) 04:26, 14 February 2011 (UTC)
Salaries
what is the salary of an engineer working for government after passing IES (Indian Engineering Services exam)? —Preceding unsigned comment added by 1.23.10.106 (talk) 15:13, 12 February 2011 (UTC)
- Determining the salary of an entry-level engineer in a government position (I presume it's India that your enquiry is based in) is difficult, as salaries can change from year to year. Additionally, public-sector roles are reorganised regularly, so the job itself may change. Perhaps this article on the Indian Engineering Service, or this link to the Union Public Service Commission of India, might be of use to you. Malusmoriendumest (talk) 08:11, 13 February 2011 (UTC)
Relationship between island size and its freshwater supply
Consider a hypothetical island somewhere in the subtropics. If you want to put a group of say 20 people on the island year-round and want them to be self-sufficient in terms of freshwater needs, how big does the island need to be to have the needed freshwater supply? I know the question is not precisely answerable, but I'd like to see some estimates that has some rational basis. Thanks. --173.49.82.30 (talk) 19:06, 12 February 2011 (UTC)
- "Subtropical" describes a zone of temperature not rain fall, so there is no answer to you question. You need to first establish the total water requirements for a 'typical' person multiply by total number of people etc. Err.. just curious: Why do you want to know this – have you discovered the World comes to an end next year and are looking for a bolt hole? --Aspro (talk) 19:26, 12 February 2011 (UTC)
- Maybe the OP meant tropical? Near the equator rainfall is regular for most of the year. Here twenty people would not need a large island to meet their water needs assuming they had reasonable equipment for capturing rain water. I can't estimate any kind of specific size, but I think it would suffice to say that in the tropics, water is unlikely to be an issue before food or building materials. --Daniel 19:38, 12 February 2011 (UTC)
- Just to add to my earlier thoughts, very small tropical islands usually don't have any naturally occurring fresh water. Rain would be the only source so collection and storage would be very important. --Daniel 19:49, 12 February 2011 (UTC)
- Maybe the OP meant tropical? Near the equator rainfall is regular for most of the year. Here twenty people would not need a large island to meet their water needs assuming they had reasonable equipment for capturing rain water. I can't estimate any kind of specific size, but I think it would suffice to say that in the tropics, water is unlikely to be an issue before food or building materials. --Daniel 19:38, 12 February 2011 (UTC)
- The questioner can use the Hawaiian Islands as an example. Size is not as important as elevation. Simply look at Oahu. One side of the island has regular rainfall. The other does not. It is due to the mountains. The wind predominantly blows from the same side. The mountains force the moist wind upward where it cools. Rain forms. For more on this, see orographic precipitation. -- kainaw™ 20:17, 12 February 2011 (UTC)
- Another example is Yakushima in Japan. It's relatively small, but also has a very high peak, so clouds bump into the island all the time and it rains almost every day. TomorrowTime (talk) 01:30, 13 February 2011 (UTC)
- The most straightforward way to evaluate this is to calculate the size of the drainage basin (which only depends on topography), and average precipitation rate for the drainage basin (which can be easily measured). Excluding ground permeability (that is - water that goes down into the ground, instead of flowing on the surface), this will approximately give you the net surface water outflow rate. This subject can be very complicated - specialists in environmental engineering and water quality management spend years trying to accurately model the water cycle for specific regions.
- The next thing to worry about is the nontrivial effect of water-pollution on a very small island. Will humans effectively manage their sewage to guarantee that it doesn't contaminate their drinking water? If so, you need to calculate how much water that would require. Will sewage from livestock, domesticated animals, or wildlife contribute to water-pollution?
- Finally, will the humans have any technology (even primitive filtration systems) to clean up polluted or microbe-infested water? If so, they can survive on much smaller quantities of water; otherwise, you will need "fast-moving" streams or rivers - this necessarily requires more water throughput. Nimur (talk) 20:54, 12 February 2011 (UTC)
Odd One out of n Resistors
Hello. There are 10 resistors, nine of which are 1 Ω each. How do I identify the odd resistor and its resistance in the least number of ohmmeter readings? I am allowed zero-resistance wires but no batteries. Thanks in advance. --Mayfare (talk) 21:20, 12 February 2011 (UTC)
- Note that the ohmmeter has a battery in it, but I expect that you are allowed to use it. Then, just divide and conquer. If you divide them into two groups of 5 resistors and connect them in series, each should read 5 ohms unless one of the piles has a resistor that is not 1 ohm. Once you identify the pile with the odd resistor, do the same. Divide into two piles and connect all the resistors in the pile in series. Continue until you have only 2 resistors. -- kainaw™ 21:25, 12 February 2011 (UTC)
- I think there may be a more efficient way to do it. At the moment this is just a hunch, because I haven't worked out all the details or verified that it actually works, but suppose you do this: First, number all the resistors, so you can distinguish them. Connect the first two resistors in parallel, and then connect that network in series with the third resistor, and then connect that whole thing in parallel to the fourth resistor, and so on, and then measure the equivalent resistance of the resulting network. You can work out what the equivalent resistance should be if all the resistors were 1 ohm; the reading you get will at least tell you whether the odd resistor is higher or lower than 1 ohm. Additionally, you can figure out things like, "If resistor 1 is the odd resistor, then its resistance must be _____; if resistor 2 is the odd resistor, then its resistance must be _____; etc." Now connect the resistors together in a different configuration (I'm being vague here, because I don't know for sure what this configuration should be), and measure the resistance again, and recompute "If resistor 1 is the odd resistor, then its resistance must be _____, etc." Perhaps it is possible to design the two configurations of resistors in such a way that, for any pair of ohmmeter readings, there is only one consistent possibility. If so, then you've solved the problem with just two ohmmeter readings. The details of this idea are left as an exercise to the reader. :-) —Bkell (talk) 21:40, 12 February 2011 (UTC)
- A guess: Perhaps the second configuration should be like the first, except that you connect resistors 9 and 10 in parallel, and then connect that network in series with resistor 8, and then connect that network in parallel with resistor 7, and so on. Will that do the trick? It seems plausible to me. —Bkell (talk) 21:49, 12 February 2011 (UTC)
- I think there may be a more efficient way to do it. At the moment this is just a hunch, because I haven't worked out all the details or verified that it actually works, but suppose you do this: First, number all the resistors, so you can distinguish them. Connect the first two resistors in parallel, and then connect that network in series with the third resistor, and then connect that whole thing in parallel to the fourth resistor, and so on, and then measure the equivalent resistance of the resulting network. You can work out what the equivalent resistance should be if all the resistors were 1 ohm; the reading you get will at least tell you whether the odd resistor is higher or lower than 1 ohm. Additionally, you can figure out things like, "If resistor 1 is the odd resistor, then its resistance must be _____; if resistor 2 is the odd resistor, then its resistance must be _____; etc." Now connect the resistors together in a different configuration (I'm being vague here, because I don't know for sure what this configuration should be), and measure the resistance again, and recompute "If resistor 1 is the odd resistor, then its resistance must be _____, etc." Perhaps it is possible to design the two configurations of resistors in such a way that, for any pair of ohmmeter readings, there is only one consistent possibility. If so, then you've solved the problem with just two ohmmeter readings. The details of this idea are left as an exercise to the reader. :-) —Bkell (talk) 21:40, 12 February 2011 (UTC)
- This is a pretty common mind-game or final-exam-question for circuit-design and related EE courses:) Divide-and-conquor is the minimum "student thought about it" answer, based on powers-of-two. There are a million variations/applications of this approach ("how many yes/no questions do you need to ask to determine which playing card someone has picked", etc.). The parallel/series-combinations approach is the really clever solution, solvable in a single measurement for certain numbers of resistors. The question is all over google (both actual science/hobbyist sites and "just tell me the damn answer to my homework question" boardd), but the parallel/series way is only on some of their answers:) DMacks (talk) 21:57, 12 February 2011 (UTC)
- Can you really solve it in a single measurement if you don't know the resistance of the odd resistor? I thought about that for a while, and I convinced myself that at least some measurements of the equivalent resistance of the series-parallel network could not lead to a unique conclusion. In particular, if one of the two "innermost" resistors is the odd one out, you have no way of telling which one. —Bkell (talk) 22:02, 12 February 2011 (UTC)
- Yes, I'm pretty sure 2 is the best for worst-case scenario or if you need to figure out if the odd one is high vs low. But it can be solved in 1 depending on where the odd one winds up. For example, solves in 2 if the odd one is R1 or R2 as you say, but solves in 1 and even know what the odd one's resistance actually is if R3 is the odd one. Is 4 completely knowable (which R#, what value) in by a single measurement unless the odd one is R1 or R2? Is this the start of a pattern where there is always only that one pair that cannot be distinguished by single measurement? DMacks (talk) 22:30, 12 February 2011 (UTC)
- Hmm, let's consider that three-resistor network. I agree that for some values of R3, a single measurement is enough to identify it. But if R3 is 1.2 ohms, for example, you will get an equivalent resistance of 0.75 ohm, which is the same equivalent resistance you'd get if R1 was the odd resistor, having a resistance of 2 ohms. So I still think, even if you get lucky and R3 is the odd resistor, you'll sometimes need at least two measurements. —Bkell (talk) 22:54, 12 February 2011 (UTC)
- Oooh good point! This is all based on vague recollections of working it out years ago, maybe I was assuming it was known if the problem stated if the odd one was high or low. DMacks (talk) 23:08, 12 February 2011 (UTC)
- Well, in the counterexample I just showed, both possible cases have a "high" resistor: the first case has R3 too high at 1.2 ohms, and the second case has R1 too high at 2 ohms. So even knowing whether the odd resistor is too high or too low isn't enough information. —Bkell (talk) 23:18, 12 February 2011 (UTC)
- Oooh good point! This is all based on vague recollections of working it out years ago, maybe I was assuming it was known if the problem stated if the odd one was high or low. DMacks (talk) 23:08, 12 February 2011 (UTC)
- And in your four-resistor network, R3 is in parallel with R4, so if one of those was the odd resistor you couldn't tell them apart. My guess is that you meant to put R4 in series with the three-resistor network. —Bkell (talk) 22:59, 12 February 2011 (UTC)
- -R4- is outside the R1/R2/R3 bracket (parallel to the whole 3-resistor circuit. Lemme try again with longer leads...(wikipedia's TeX doesn't seem to support boxes). DMacks (talk) 23:08, 12 February 2011 (UTC)
- Yes, I understand where R4 is. It is still in parallel with R3. The network as a whole has three branches in parallel: one branch consists of R1+R2, the second consists of R3, and the third consists of R4. The network you have drawn is electrically equivalent to . The resistors R3 and R4 are perfectly symmetric in this network. I think what you want to draw is , where R4 is in series with the three-resistor network. —Bkell (talk) 23:14, 12 February 2011 (UTC)
- Ah yeah. There was some pattern for extending each next resistor. And there was also definitely some more specific parameter on the odd-one, since even now you could swap in any of them and get the same result by different choices of the odd-one's value. I'm gonna quit trying to work out the details, been too long since I've forgotten the cleverness:( DMacks (talk) 04:04, 13 February 2011 (UTC)
- Yes, I understand where R4 is. It is still in parallel with R3. The network as a whole has three branches in parallel: one branch consists of R1+R2, the second consists of R3, and the third consists of R4. The network you have drawn is electrically equivalent to . The resistors R3 and R4 are perfectly symmetric in this network. I think what you want to draw is , where R4 is in series with the three-resistor network. —Bkell (talk) 23:14, 12 February 2011 (UTC)
- -R4- is outside the R1/R2/R3 bracket (parallel to the whole 3-resistor circuit. Lemme try again with longer leads...(wikipedia's TeX doesn't seem to support boxes). DMacks (talk) 23:08, 12 February 2011 (UTC)
- Hmm, let's consider that three-resistor network. I agree that for some values of R3, a single measurement is enough to identify it. But if R3 is 1.2 ohms, for example, you will get an equivalent resistance of 0.75 ohm, which is the same equivalent resistance you'd get if R1 was the odd resistor, having a resistance of 2 ohms. So I still think, even if you get lucky and R3 is the odd resistor, you'll sometimes need at least two measurements. —Bkell (talk) 22:54, 12 February 2011 (UTC)
- Yes, I'm pretty sure 2 is the best for worst-case scenario or if you need to figure out if the odd one is high vs low. But it can be solved in 1 depending on where the odd one winds up. For example, solves in 2 if the odd one is R1 or R2 as you say, but solves in 1 and even know what the odd one's resistance actually is if R3 is the odd one. Is 4 completely knowable (which R#, what value) in by a single measurement unless the odd one is R1 or R2? Is this the start of a pattern where there is always only that one pair that cannot be distinguished by single measurement? DMacks (talk) 22:30, 12 February 2011 (UTC)
- Can you really solve it in a single measurement if you don't know the resistance of the odd resistor? I thought about that for a while, and I convinced myself that at least some measurements of the equivalent resistance of the series-parallel network could not lead to a unique conclusion. In particular, if one of the two "innermost" resistors is the odd one out, you have no way of telling which one. —Bkell (talk) 22:02, 12 February 2011 (UTC)

- I'm thinking that the circuit I diagram at right should work, provided that you know the unknown resistance, and you're not so unlucky as to put it in one of the spots I failed to make unique (is there a better way to do it? I always end up with some odd resistors out. But I think that if you scramble the resistors and measure twice it should work. But the circuit I draw does bring back fearful memories of the dreaded Wheatstone Bridge from bygone days... and for this to work I'd have to figure out V2, V3, V4, V5... Sigh, let's see, Kirchhoff's circuit laws... Wnt (talk) 06:58, 13 February 2011 (UTC)
Good God, but I'm coming up with stuff like "v5 = (-v1/R1 - v1/R2 +v2/R4 +v2/R1 +v2/R3 +v3/R5+v3/R2+v3/R6-v4/R3)/(1/R4 + 1/R5)". And I still have v4, v3, v2, and v1 to solve for. Wnt (talk) 07:40, 13 February 2011 (UTC)

- The kind of network I was suggesting is shown to the right. It is built by connecting resistors D and E in parallel, and then connecting C to that network in series, and then connecting B to that network in parallel, and then connecting A to that network in series; it is built up one resistor at a time by alternating series connections and parallel connections. I believe that no two resistors here are interchangeable except the two innermost ones (D and E). This kind of network has the advantage of being series-parallel, unlike the "grid" of resistors Wnt is building; so you can analyze it using just simple series and parallel reductions, rather than having to perform Y-Δ transforms. I conjecture (though I haven't proved this) that if you build a second network like this, but connect the resistors in the reverse order (swap A and E, swap B and D, C stays where it is) then the measurements of the equivalent resistances of the two networks will identify the odd resistor and its value. —Bkell (talk) 08:02, 13 February 2011 (UTC)
Wouldn't you need 3 readings in the worst case? Say you take 1 reading for the first five resistors. But those resistors are identical. You take another 2 readings to find the odd resistor in the other set of five resistors. I may be confused. --Mayfare (talk) 23:20, 13 February 2011 (UTC)
- (To Mayfare:) Oh, yeah, sorry for the confusion. The picture there is only for five resistors; that's only because I had already drawn that picture for a paper I wrote several years ago, so it was easy to just upload what I had rather than drawing a new one. So my explanation was for a puzzle where you just have five resistors. But the idea extends to any number of resistors. If you have ten resistors in all, then just continue that construction: add the next resistor in parallel with these five, and then the sixth resistor in series with that, and so on, adding each resistor one at a time and alternating series and parallel connections. Then find the equivalent resistance of that whole 10-resistor network. Now build the network again, but use the resistors in the reverse order (so the "innermost" resistors from the first network become the "outermost" resistors in the new network, and vice versa), and measure the equivalent resistance of that network. My conjecture is that these two measurements will uniquely identify the odd resistor and its value (ignoring the issues about measurement errors and resistor tolerances that Gr8xoz raises below—I'm assuming that since we are allowed to use zero-resistance wires we also have a perfect ohmmeter and perfect one-ohm resistors). —Bkell (talk) 00:22, 14 February 2011 (UTC)
- This method is very sensitive to measurement errors and errors in the resistance of the nine resistors that are suposed to be 1 ohm. 10 resistors connected in this meaner where one of the innermost resistors have a resistance of 2 ohm and the rest are 1 ohm have an total resistance of 0.618055 ohm while if it is the third innermost resistor the resistance becomes 0.618320 ohm. With normal ohm meters and resistors this is easily masked by the errors in measurement and the nine "1 ohm" resistors. I have not analysed the "grid" suggested by Wnt but I would expect it to have similar problems. In order to answer the question we need to know the measurement error, the allowed errors in the resistances and the minimum allowed difference between the odd resistance and 1 ohm. Gr8xoz (talk) 10:57, 13 February 2011 (UTC)
February 13
Hill, James B. (1945). Autobiography
An article references "Hill, James B. (1945). Autobiography. Raceland, Louisiana, USA: James B. Hill. pp. 200". How can I get more information on this autobiography? —Preceding unsigned comment added by 66.97.56.130 (talk) 01:34, 13 February 2011 (UTC)
- You could try your local library; they're unlikely to have a copy themselve, but they do they have experts in locating books. But it is not certain that it is findable at all. The format of your reference indicates that the book is self-published (the name that comes after the place of publication is that of the publisher), so it may be a small run that has never been catalogued by a general library. The book does not seem to exist in the Library of Congress's on-line catalog. –Henning Makholm (talk) 02:52, 13 February 2011 (UTC)
- Also, this would be better asked on the humanities desk. –Henning Makholm (talk) 02:52, 13 February 2011 (UTC)
Time when Sun is highest in the sky
On any given day, is the Sun at its highest in the sky at exactly the same time in all places with the same longitude? (By "same time" I mean same time as measured by a single reference clock. Ignore any issues to do with local time.) I've always assumed, without thinking too much about it, that the answer was "yes", but now I'm beginning to doubt it. 86.183.3.100 (talk) 04:02, 13 February 2011 (UTC)
- Yes, local noon has to happen simultaneously on an entire meridian. Noon happens at the moment the abstract plane that contains the Earth's axis and the place in question sweeps through the center of the Sun. But this plane is the same for all places on the meridian, so their noons are the same.
- (I'm assuming you're not interested in things like relativistic aberration of the sunlight due to the different rotational speeds on points at different distances from the axis. Not sure the effect is measurable anyway). –Henning Makholm (talk) 04:49, 13 February 2011 (UTC)
- One possible exception is in the Arctic or Antarctic, where, depending on the time of year, the Sun may not come up at all that day. So, if there is no Sun at all, the time when it is highest in the sky is undefined (unless, I suppose, you consider that to be when the Sun is closest to, but still below, the horizon). StuRat (talk) 04:10, 14 February 2011 (UTC)
Thanks for the replies. I am trying to calculate the declination of the Sun as seen from Earth at different latitudes, and at different times of year, using a simplified model. The model assumes that the Earth’s orbit is circular and ignores various other complications such as precession. I know that the model is not exact, but it does not seem feasible that making it exact by adding ellipticity and the other tweaks will exactly eliminate the discrepancy that I observe. In the model:
k is number of Earth rotations per year, which I’m assuming is 366.25
alpha is angle of Earth’s tilt, which I’m assuming is 23.5 deg.
a is the time at which we measure the Sun’s declination, in years. a = 0 is the moment of the December solstice in some year (for me it doesn’t matter which one). a = 1 is the moment of the December solstice one year later.
phi is the latitude on Earth from which we observe the Sun
I assume that the point from which we observe the Sun is on the longitude that is exactly facing away from the Sun (i.e. midnight) at a = 0.
I set up a coordinate system whereby the x and y axes lie in the plane of the Earth’s orbit, with the x-axis pointing in the direction from the Sun to the Earth at a = 0, and the y-axis pointing in the direction that the Earth is (instantaneously) moving in its orbit at a = 0. The z-axis points North out of the orbital plane.
To find the declination of the Sun, I first calculate the direction (unit) vector (x,y,z) from the centre of the Earth to the viewpoint on the Earth’s surface at time = a. I do this as follows:
At a = 0, without yet accounting for the Earth’s tilt, we know (x,y,z) = (cos(phi), 0, sin(phi)).
Then, I account for the Earth’s rotation at time = a by rotating vector (x,y,z) about the z-axis (anticlockwise as looking from positive z to negative z) by an angle 2*pi*k*a.
Then I account for the axial tilt by rotating (x,y,z) about the y-axis (clockwise as looking from negative y to positive y) through angle alpha.
Then I calculate theta, the angle between the resulting vector (x,y,z) and the vector from the centre of the Earth to the Sun at time = a, which I make to be (-cos(2*pi*a), -sin(2*pi*a), 0).
Finally I calculate: Sun’s declination = pi/2 - theta
According to my calculations, this results in:
declination = -pi / 2 + acs(cos(alpha)*cos(phi)*cos(2*pi*a)*cos(2*pi*k*a) + cos(phi)*sin(2*pi*a)*sin(2*pi*k*a) + sin(alpha)*sin(phi)*cos(2*pi*a))
The trouble is that I can’t see either numerically or analytically how the maxima of this (w.r.t a) are independent of phi. I get very slight variations.
What am I doing wrong? Thanks to anyone who has got this far! 81.159.104.144 (talk) 14:35, 13 February 2011 (UTC)
- Hmmm, actually you are right and we were wrong. I get the same equation as you do.
- What went wrong in our intuitive understanding was that we confused "Sun highest in the sky" with "Sun appears due south". They are not exactly equivalent. The "due south" moment (which is simultaneous along an entire meridian) is when the fixed star that the Sun is momentarily in front of has its highest elevation. At that time, this star moves horizontally, but at the same time the Sun is moving along the ecliptic relative to the fixed stars, and this movement generally has a north-south component. So at the "due south" moment, the Sun is actually still moving slightly upwards in the sky, or already moving downwards, depending on which hemisphere we are in, meaning that "highest solar elevation" noon does vary very slightly along the meridian.
- This also helps clear up the apparent discontinuity at the poles: When we move closer to the pole, in the limit they daily variation in the Sun's elevation gets much smaller than the variation due to the ecliptic movement. So on any meridian, the "highest elevation" moment during the summer solstice day must converge towards the exact solstice moment as the latitude approaches 90° exactly. –Henning Makholm (talk) 17:50, 13 February 2011 (UTC)
- Hi Henning, thank you very much for taking the time to look at this. I'm delighted that you now get the same result as me; staring at these equations has been driving me nuts! 86.179.118.226 (talk) 18:44, 13 February 2011 (UTC)
- Henning Makholm wrote" "The "due south" moment (which is simultaneous along an entire meridian)" You are forgetting about the part of the earth south of the Tropic of Capricorn where the noon sun is due north. Between the Tropic of Capricorn and the Tropic of Cancer the sun may be north or south at noon - depending on the date - and twice a year it will be overhead. Roger (talk) 21:15, 14 February 2011 (UTC)
agate spatula
Why is the instrument named agate spatula?? what is the reason 4 it?? — Preceding unsigned comment added by Suprithmurthy (talk • contribs) 04:31, 13 February 2011 (UTC)
Name for an auction method
My friend described a kind of auction in game theory/economics to me, but I don't know what it's called, and it doesn't seem to be any of the ones described in Auction theory. Here's how it happens:
The seller sets a secret reserve price for the item. Each bidder puts a secret bid in an envelope. The envelopes are opened, and if any bid meets or exceeds the reserve price, the highest bidder wins, but that bidder pays only the reserve price, rather than their bid. If no bid meets the reserve, the item remains unsold.
What is this kind of auction called? —Keenan Pepper 05:42, 13 February 2011 (UTC)
- How could this auction work? After the auctioneer has all the bids, he would secretly sneak a peek in the envelopes of a few predicted highest bidders, and set the "reserve price" to just below the highest bid he sees. Alternatively, he'd tell a friend what the reserve price was, and that friend, confident that it was fairly low, could submit an absurdly high bid and clinch the item. Even an honest auction of this type leaves the seller in the peculiar position of accepting less money than the buyers are willing to bid. I have a hard time believing this is actually done - is this a real auction type or just a thought experiment for game theory? Wnt (talk) 06:24, 13 February 2011 (UTC)
- Right, it's not a practical method for real auctions. But what is it called? —Keenan Pepper 07:12, 13 February 2011 (UTC)
- If it's not practical, then why do you suppose anybody would bother to think of a name for it? –Henning Makholm (talk) 07:23, 13 February 2011 (UTC)
- Oh, don't be so naive. Lost of people spend their time thinking about unpractical things. Dauto (talk) 07:44, 13 February 2011 (UTC)
- Sure, but things don't usually get named until they've been thought about by several people (who know about the previous thoughts). This is the kind of auction idea that would get thought about once, rejected as impractical, and then not thought of again. --Tango (talk) 21:22, 13 February 2011 (UTC)
- Oh, don't be so naive. Lost of people spend their time thinking about unpractical things. Dauto (talk) 07:44, 13 February 2011 (UTC)
Just to be sure...your friend isn't just getting themselves a bit muddled? What they describe sounds quite like a Vickrey auction - except that very last bit...that is to say in a `Vickrey auction' the winning person doesn't pay what they bid, they pay whatever the second highest bid was. Could it be that this is what they were describing? In the auction idea you describe I can't quite figure out what incentive people have to bid low assuming they want to win the item? ny156uk (talk) 09:14, 13 February 2011 (UTC)
- Right, exactly. In fact I believe in this kind of auction the bidders have an incentive to bid their true value, which is why I think it's probably been discussed in a game theory context. —Keenan Pepper 17:48, 13 February 2011 (UTC)
I agree that this sounds like a second-price auction. As noted above, the equilibrium in this auction is for all players to bid their true valuations (assuming the standard economic utility framework). The more familiar first-price auction provides an incentive for the winning player to over-bid (the "winner's curse.").
As to the auction described, it seems it would be unworkable if more than one bidder valued the item greater than the reserve price. Each bidder will attempt to outbid the other, without constraint (since they only pay the reserve price no matter how high they bid). There is no Nash Equilibrium to this game since the strategy space is infinite and unbounded. It's a little like playing poker for a set number of dollars per hand and an infinite bankroll; the strategic possibilities would break down very quickly. —Preceding unsigned comment added by 12.186.80.1 (talk) 16:07, 15 February 2011 (UTC)
- It seems like nobody even READ what I wrote (except Dauto). The reserve price is SECRET. Each bidder ONLY BIDS ONCE. —Keenan Pepper 17:33, 18 February 2011 (UTC)
Smallpox Vaccine
What is the big needle used for when giving a smallpox vaccine?

Also, why do people who have gotten the vaccine have a scar on their shoulder? Scar on Shoulder —Preceding unsigned comment added by 76.169.33.234 (talk) 07:53, 13 February 2011 (UTC)
- Sorry - I took the liberty to change your figures over to the standard thumbnail format, because they were too big and foul up display of the Refdesk.
I didn't know that by using a single bracket http link to the thumbnail you can get them to display,but the humdrum way is more practical. Wnt (talk) 07:59, 13 February 2011 (UTC)- Thanks! I couldn't figure it out, first it was too big, then it was just links. Looks much better now. —Preceding unsigned comment added by 76.169.33.234 (talk) 08:01, 13 February 2011 (UTC)
- You may be interested in Smallpox vaccine#Post-eradication vaccination Nil Einne (talk) 08:17, 13 February 2011 (UTC)
- The reason for the needle's shape and how its used is given in the article Bifurcated needle.--Aspro (talk) 11:23, 13 February 2011 (UTC)
- Thanks for the info. The article states that the established method was to do it in the left arm, was there any particular reason for this? Why not do it someplace else where the scar that is left is not visible? Is the permanent scar that is left because of the vaccine or because of the needle? —Preceding unsigned comment added by 76.169.33.234 (talk) 11:53, 13 February 2011 (UTC)
- The scar results because the vaccine is a live virus, which multiplies at the site of introduction (in the cells around the skin puncture). One's immune system then kicks in and confers immunity which prevents the virus spreading and causing more 'pox marks.' This local infection is what causes a scab to form, which eventual drops off. The needle just needs to puncture the skin enough to see a little blood. This is because unbroken skin is a very good barrier and may prevent the virus from successfully invading the live skin cells below. The tiny puncher marks on their own, would otherwise heal without a trace. So it is the infection that leaves the scare.
- People who have had small pox, very often carry pox marks for the rest of their lives.
- The inoculation is performed on the opposite side to the dominant hand because as it is a live virus and a real irritating infection, the site is commonly tender and sore -and can itch like mad. In a few people, it can ache so much that they like to have the arm in a sling. Hence the preference for the arm on the opposite side. It is for this reason too that it is better on the arm than the leg. Also, there is the practical aspect that in an inoculation drive, it is more convenient to have people (maybe a thousand or more in a day) form a line with their sleeves rolled up. One doesn't have to lean too far down to reach each arm. You never find medical students picking grapes on their vaccation – too much bending!--Aspro (talk) 15:32, 13 February 2011 (UTC)
- If the smallpox vaccine were still given today routinely like it was in the past, do doctors now have something that they can give to the patients to minimize the scars? --173.49.14.8 (talk) 04:30, 14 February 2011 (UTC)
- In the past the scars were not considered an issue because almost everybody had one, so I would image they would be dismissed in the same way today. The only treatment I can think of, that may reduce scaring, would be to stop the blister from drying out and forming a hard scab. This could be done by applying silicone scar sheets and then continuing for some time after the dead skin sloughs off, with the topical application of a suitable skin gel. Other treatments such as ex-foliation of the skin etc., to encourage it to regrow smooth is I think – vanity going too far.--Aspro (talk) 11:37, 14 February 2011 (UTC)
- If the smallpox vaccine were still given today routinely like it was in the past, do doctors now have something that they can give to the patients to minimize the scars? --173.49.14.8 (talk) 04:30, 14 February 2011 (UTC)
- Note that the smallpox vaccine isn't the only vaccine which produces a scar. The Bacillus Calmette-Guérin is also know for the scarring although that is usually applied to the upper arm and with a more normal hypodermic needle (at least it was in Malaysia) AFAIK. The BCG uses attenuated live bacteria however a live vaccine obviously doesn't guarantee prominent/significant scarring as it depends on specific vaccine and the bodies response thereof, for example I don't believe the MMR vaccine is notable for any particular scarring. Nil Einne (talk) 07:24, 14 February 2011 (UTC)
Vasectomy: Potential post-operative hormonal changes
The standard article on vasectomy states that after the procedure, "Sperm are still produced by the testicles, but they are broken down and absorbed by the body." Several external articles (which may or may not be objective) put the "average time" for spermatozoa to be reabsorbed into the body after production at around 40 days (but their cited source is obscure). Many papers are available on vasectomy itself, post-operative complications and chronic pain, but I am unable to determine whether any studies have been conducted on post-operative hormonal changes. My question pertains to whether male hormonal levels (specifically those of androgens such as androstenedione and testosterone) are likely to increase measurably after vasectomy, given that sperm cells and their constituent elements are no longer being expelled from the body, but broken down and re-used. Any study or article that shows measurable change (of even the smallest degree) might be relevant; failing that, even anecdotal evidence might be of use, provided that its evidential value is reinforced by multiple instances. Consulting urological surgeons has not yielded results, since their speciality might be said to lie in the performance of the procedure, not its effect on the delicate balances of blood chemistry.
Malusmoriendumest (talk) 08:05, 13 February 2011 (UTC)
Temperature and wavelengths
Is it possible to contain all wavelengths/frequencies at a specific temperature? For example does Sun emit frequencies in the range of 0 to ∞ Hz, and does our body do the same thing?--Email4mobile (talk) 08:33, 13 February 2011 (UTC)
- blackbody radiation 83.134.173.228 (talk) 10:05, 13 February 2011 (UTC)
- I think I couldn't find a clear answer in that article. Let me give a direct example: Is heating iron to 1000 °C associated with Gamma rays emissions or Radio frequency waves?--Email4mobile (talk) 10:18, 13 February 2011 (UTC)
- I was reluctant to say anything more because I don't want to risk saying anything false about extremely high energy photons. Anywaw, as far as black body radiation applies, any body at nonzero temperature emits radiation at all wavelengths, so the answer to your original question is yes (you personally emit some extremely small amount of gamma rays). Nevertheles, most of the radiated energy goes into radiation at a certain frequency range that is a function of the body's temperature. So 1000°C emits mostly visible light and infrared, but it also emits some negligibly small amount of radio waves and gamma rays. 83.134.173.228 (talk) 11:07, 13 February 2011 (UTC)
- The article on Planck's law shows the rather slewed bell curve of the spectral output verses temperature. It is the peak of the curve that corresponds to a specific temperature.--Aspro (talk) 11:37, 13 February 2011 (UTC)
- I haven't worked out the numbers for when this would happen, but if you have a cold enough object and you are interested in a short enough wavelength then, while technically some radiation at that wavelength will be emitted by that object, it may work out as less than one photon during the entire age of the universe. That can be interpretted as essentially not emitting anything at that wavelenght. --Tango (talk) 21:31, 13 February 2011 (UTC)
- I was interested in this question, and my back-of-envelope calculation suggests that for normal-sized black-body objects at around room temperature, this cutoff point, beyond which a total (over all wavelengths) of less than one photon is emitted during the entire age of the universe so far, lies somewhere in the visible spectrum. (Don't rely on this answer though ... I could have done something drastically wrong!) 86.160.216.197 (talk) 21:40, 14 February 2011 (UTC)
Thanks for the answers. Indeed I was thinking something similar and wanted to share the question before I submit the answer in Arabic for someone asked in Google Answers (Google opened Arabic service less than two years ago). I can understand that even if there were gamma or RF waves, they would be negligible in the hole spectrum at temperature ranges less than several thousands °C.--Email4mobile (talk) 22:45, 15 February 2011 (UTC)
which software
please any experienced person help me .. which software i should i use to model a disintegration of nuclear fuel rod(fission process).please give any other useful regarding its modelling —Preceding unsigned comment added by 59.93.130.74 (talk) 09:33, 13 February 2011 (UTC)
- There are different Nuclear fuel cycles. This article may help you focus in on the one that suits your application best. Then you can start looking for the software that models that cycle. Thorium is thought to be the next big thing in reactor design. --Aspro (talk) 11:42, 13 February 2011 (UTC)
- Perhaps User:Rocketshiporion can help? Their interest seemed to be in the nuclear weapon side but they may have picked up something in their research that may be of use Nil Einne (talk) 16:08, 14 February 2011 (UTC)
- If you mean the physical disintegration of the nuclear fuel rod (which AFAIK shouldn't happen in a properly designed reactor), see Field Precision's GamBet Monte Carlo Suite. If OTOH you mean modeling the operation of a nuclear reactor, you probably need something like this, but unfortunately it is not commercially available. Rocketshiporion♫ 05:12, 17 February 2011 (UTC)
physics
what is horizon of a time machine? — Preceding unsigned comment added by Sanandnps (talk • contribs) 14:38, 13 February 2011 (UTC)
- You can't title your question "Physics" and then ask about time machines. Since time machines only exist in fiction, the entertainment forum might be a better place for your question. Dauto (talk) 15:46, 13 February 2011 (UTC)
- Why answer so rudely? 1) Time travel is a very real topic in physics, and the time travel article has dozens of references from peer-reviewed physics journal articles, 2) if you don't have an answer, why post? — Sam 67.186.134.236 (talk) 18:17, 13 February 2011 (UTC)
- That depends. Is the hypothesized time machine traveling forward or backward in time? If traveling forward in time (faster than normal), one way to do that would be to hang out just outside the event horizon of a black hole, as mentioned in Time travel#Time travel to the future in physics. Traveling back in time may or may not be possible, depending on whether closed timelike curves actually exist. The boundary of a set of closed timelike curves is a Cauchy horizon, which is touched on lightly in Time travel#Other approaches based on general relativity. Red Act (talk) 16:21, 13 February 2011 (UTC)
- By the way, even if travel along a closed timelike curve is possible (and there are some very strong constraints on that possibility, such as Hawking's constraint prohibiting a compactly generated Cauchy horizon in a region where the weak energy condition is satisfied), the nature of doing that would be very different from the type of time travel that's pretty much always depicted in fiction, in which the time machine or time traveler has a discontinuous world line. A discontinuous jump in a world line like that is just fiction. Red Act (talk) 17:38, 13 February 2011 (UTC)
- Dang, I just wish for the days when all you had to do was push this little button and you appeared in a futuristic city filled with Eloi. Crimsonraptor | (Contact me) Dumpster dive if you must 18:46, 13 February 2011 (UTC)
- By the way, even if travel along a closed timelike curve is possible (and there are some very strong constraints on that possibility, such as Hawking's constraint prohibiting a compactly generated Cauchy horizon in a region where the weak energy condition is satisfied), the nature of doing that would be very different from the type of time travel that's pretty much always depicted in fiction, in which the time machine or time traveler has a discontinuous world line. A discontinuous jump in a world line like that is just fiction. Red Act (talk) 17:38, 13 February 2011 (UTC)
Pig feed
What are domesticated pigs at pig farms fed? (historical info would be interesting as well)Mintyf (talk) 15:41, 13 February 2011 (UTC)
- It's unsourced and not terribly detailed, but we do have some information in the second paragraph of Intensive_pig_farming#Intensive_piggeries and at Sty#Slopping_the_Hogs. --Allen (talk) 18:24, 13 February 2011 (UTC)
- They're fed pelletized grain. --Sean 15:38, 14 February 2011 (UTC)
- For historical information, Toilets_in_Japan#History states that:In Okinawa, the toilet was often attached to the pig pen, and the pigs were fed with the human waste product. This practice was banned as unhygienic after World War II by the American authorities....though I don't believe that practice was widespread.Smallman12q (talk) 19:28, 14 February 2011 (UTC)
What is this plant?
http://img.skitch.com/20110213-mykikb758gb8g8si9q5kh865gi.jpg
Hi all,
Does anyone know what this plant is? We were thinking it was a Wandering Jew, but now I don't think it looks that much like one. Thanks so much! — Sam 67.186.134.236 (talk) 18:07, 13 February 2011 (UTC)
- I know it as a Wandering Jew too, but I think it's one of the Tradescantia family. --TammyMoet (talk) 18:28, 13 February 2011 (UTC)
- Maybe specifically Tradescantia spathacea—cf. this Flickr image. Deor (talk) 18:34, 13 February 2011 (UTC)
- Thanks! — Sam 67.186.134.236 (talk) 21:15, 13 February 2011 (UTC)
- Maybe specifically Tradescantia spathacea—cf. this Flickr image. Deor (talk) 18:34, 13 February 2011 (UTC)
Low-density solids/liquids?
Are there any solids or liquids that are less dense than air? --75.15.161.185 (talk) 21:01, 13 February 2011 (UTC)
- Our article Aerogel has some information on this topic:
- "The world's lowest-density solid is a silica nanofoam at 1 mg/cm3, which is the evacuated version of the record-aerogel of 1.9 mg/cm3. The density of air is 1.2 mg/cm3."
- So it seems there are solids lighter than air, but they're highly porous materials.
- Ben (talk) 21:09, 13 February 2011 (UTC)
- So why don't they float off into space? --75.15.161.185 (talk) 21:16, 13 February 2011 (UTC)
- I think "evacuated version" means what you get when you put it in a vacuum. In air, it has a density higher than air so doesn't float away. In a vacuum, there is nothing for it to float in. --Tango (talk) 21:34, 13 February 2011 (UTC)
- so then in other words, because the structure of an aerogel is always "thin strands of open-cell structure of something, with air inbetween," the answer is no? 65.29.47.55 (talk) 22:58, 13 February 2011 (UTC)
- I think "evacuated version" means what you get when you put it in a vacuum. In air, it has a density higher than air so doesn't float away. In a vacuum, there is nothing for it to float in. --Tango (talk) 21:34, 13 February 2011 (UTC)
- So why don't they float off into space? --75.15.161.185 (talk) 21:16, 13 February 2011 (UTC)
So are there any solids or liquids that are less dense than air when in the atmosphere (i.e. not in a vacuum)? --75.15.161.185 (talk) 23:01, 13 February 2011 (UTC)
- I think that you should relax your standards to allow for any gas, and it will be hard to find such a case anyway. If you do this, then a gas like sulfur hexafluoride (or for those willing to put up with nasty nasty HF, tungsten hexafluoride) can be up to ten times denser.
- Now an aerogel is not "truly" solid throughout; it's a sort of mass of fibers. If you allow something not solid throughout, you might as well allow a boat. Which brings us to [3], which is about as close as I think you're going to get to what you want. Wnt (talk) 00:01, 14 February 2011 (UTC)
- Instead of going with another gas, you could go with more pressure. This would compress air more than most liquids or solids, thus making them more likely to float. StuRat (talk) 03:55, 14 February 2011 (UTC)
- I think the question is nonsensical, imagine asking the opposite: is there a gas more dense then solid? Obviously not; if it was more dense, then it would BE solid. Vespine (talk) 04:52, 14 February 2011 (UTC)
- I don't think that follows: for example liquid Mercury is much more dense than solid Sodium at room temperature. AndrewWTaylor (talk) 09:25, 14 February 2011 (UTC)
- The question and most of the discussion is about "solids and liquids" vs gases, not "solids vs liquids". Solids and liquids generally do have similar densities (molecules just less organized and a little less tightly attached to each other in liquids), whereas gases are generally orders of magnitude less dense (molecules highly spaced) at normal pressures and temperatures. DMacks (talk) 16:44, 14 February 2011 (UTC)
- I don't think that follows: for example liquid Mercury is much more dense than solid Sodium at room temperature. AndrewWTaylor (talk) 09:25, 14 February 2011 (UTC)
- I think the question is nonsensical, imagine asking the opposite: is there a gas more dense then solid? Obviously not; if it was more dense, then it would BE solid. Vespine (talk) 04:52, 14 February 2011 (UTC)
- One thing worth noting is that the definition of a "solid" is a wee bit unclear. The old rule-of-thumb "conforms to the shape of its container" (like water becomes a cylinder when poured into a glass) becomes a bit weird for gels, infusions, and glasses (silica glass has a barely-measurable viscosity). Molecularly, one might call a solid as being a stable crystal lattice, of which there are many configurations like the sorts in the phase diagram of water/ice, while polymers and glasses would be amorphous solids (or, in physics, soft condensed matter).
- The point is that aerogel is a solid as most would think of it, while a highly porous object, like the very light pumice rock, is cheating a bit (especially if we filled the holes with helium and sent it up like a zeppelin. But if you want a nice crystal structure, then here's a fun exercise: check out the [formula for theoretical density of a crystal] and compare it to the ideal gas law to see if you can get your very low-density configuration of some light atom or molecule - the point of the ideal gas law is it sets an upper limit on your solid's volume. SamuelRiv (talk) 23:39, 14 February 2011 (UTC)
- For a theoretical solid you could try solid dipositronium, unfortunately it decays too fast to be realised, but it would be lighter than air and solid, but at cryogenic temperatures. Graeme Bartlett (talk) 23:52, 14 February 2011 (UTC)
February 14
Sulfur hexafluoride boat
If you had a large enough tank filled with SF6, could a boat with a person in it float on it? Would you be able to move by paddling with the oars? --75.15.161.185 (talk) 00:19, 14 February 2011 (UTC)
- Yes and yes, though the scales would need to be rather large and your person would need breathing gear for safety. For the latter part particularly, consider the general nature of propellor-driven aircraft. — Lomn 00:41, 14 February 2011 (UTC)
- You'll need much more gas than Mythbusters used. Clarityfiend (talk) 00:52, 14 February 2011 (UTC)
- From the Sulfur hexafluoride, we have an SF6 density of 6.12 g/L or 6.12 kg/m3. From Density of air we have 1.29 kg/m3. The difference gives us a net of 4.83 kg/m3 buoyancy. If we needed to float 50kg (say a 40kg person in a 10kg boat), the boat would need to displace about 10.4 m3. Contrast with water displacement, where only about 0.05 m3 (50 liters) need be displaced to float that same 50kg. -- Tom N (tcncv) talk/contrib 01:10, 14 February 2011 (UTC)
- You'll need much more gas than Mythbusters used. Clarityfiend (talk) 00:52, 14 February 2011 (UTC)
- I would just mention that, while the videos look very cool, sulfur hexafluoride is a very powerful greenhouse gas. I'd love to try the thing where you breathe SF6 and talk (it's the flip side of the helium thing) but I'm not sure I can justify it ethically. --Trovatore (talk) 01:13, 14 February 2011 (UTC)
- Wow, you're not kidding! 23,900 times more efficient than CO2 on a 100-year time scale, 32,600 times more effective on a 500-year time scale. (Though at least that's not by weight!, but by parts per billion, which according to Parts-per_notation#Air_measurements seems to be a volume-based measurement.) My, I wonder if you can find sulfur and fluorine on Mars...[4]
- In terms of size, this would be more like and airship than a boat. The volume required to lift a person would still be much lower than helium baloons in air, though. — Preceding unsigned comment added by Roberto75780 (talk • contribs) 12:41, 14 February 2011 (UTC)
Youtube video - mechanical glitch, other artifact or mysterious unreflective object?
Hi. I recently came across this Youtube video showing images of a dark object detected by STEREO but according to an astronomer referred to in the comments is not visible on LASCO. I would be interested to know what it is. I realize the video description and annotated text could be exaggerated and jumping to only one conclusion but can a glitch persist for a month and change in size? Thanks. ~AH1(TCU) 00:21, 14 February 2011 (UTC)
- If you want to be taken even remotely seriously, do not get your STEREO data from Youtube.com. NASA makes the STEREO data available to the public at the official spacecraft webpage, STEREO, hosted at Goddard Space Flight Center. You can download the official data. If a "glitch", image-processing artifact, or astronomical observation of any significance were present in the data, it would not be reported through pseudoscience-crank-videos on Youtube.
- Although, if NASA was in on the conspiracy, they might have distorted the images in the official database. We can only presume that if it is the case, and NASA has covered up these glitches by doctoring the official data, that the author of the Youtube video was able to independently acquire the unadulterated raw video, with glitches intact, by operating his own Deep Space Network-sized ground stations; and that he has the scientific expertise to interpret and analyze the images and ascertain the presence of a mystery-object. As an anonymous Youtube-author, though, the uploader of that video has a bit less credibility than NASA.
- If you are actually interested in the scientific explanation for image artifacts, NASA's official page has an entire set of explanations for each type of artifact on each instrument on the STEREO spacecraft. Image Artifacts, from NASA, with various subpages.
- Here are some nice examples of internal reflections of Venus. These sorts of optical artifacts are very common. If you've ever photographed through a fancy compound optics system, you know how much of a problem internal refractions/reflections/distortions can be. I can personally attest to some funny optical trickery back in December while I was photographing Venus in the early morning. I could have sworn I saw a spurious bright spot - a "UFO," or a moon, hovering just off the limb of Venus. So here was this bright shiny object that I could see, plain as day through my eyepiece, but was completely immune to being photographed! Magic! Or perhaps alien technology! What actually had happened was that my camera, having been sitting right next to my warm cup of coffee, had developed some "fog" or dew on one of its internal optical surfaces (probably the mirror) while I changed lenses; and I got a very nice bright spot that was showing up only through my eyepiece (and never in my photographs). After a few minutes of thermal acclimation, the foggy layer dissipated and my "UFO" disappeared (and I was unable to claim discovery for a moon of Venus). Nimur (talk) 00:58, 14 February 2011 (UTC)
- (The specific "glitch" reported in the Youtube video was an example of the long-exposure noise reduction algorithm - it is a digital postprocessing artifact. This effect only shows up on certain data-products. Scientists studying STEREO data will usually obtain low-level data products (the rough equivalent of "shooting raw images") and apply a smarter noise-reduction method. I apologize if my earlier anecdote implied that the "dark spot" was some type of optical effect - I only meant to emphasize that astrophotography is full of "spurious data." Nimur (talk) 13:48, 14 February 2011 (UTC)
- Nimur, that is an outstanding response. Thank you for taking the time to write it, and I especially enjoyed the artifact links you included. You are a credit to your species. The Masked Booby (talk) 00:58, 15 February 2011 (UTC)
Antibacterial gels vs iodine
Hi all,
Is there any difference between using antibacterial/antiseptic gels such as Neosporin vs. old-fashioned iodine (or even rubbing alcohol)? Is one better in some cases than in others? Thanks! — Sam 63.138.152.135 (talk) 14:49, 14 February 2011 (UTC)
- Non-iodine is better in cases where the patient is allergic/sensitive to iodine. --Sean 15:45, 14 February 2011 (UTC)
- And conversely for patients allergic/sensitive to neomycin. Iodine in general can some adverse effects (scarring and interference in healing) depending on formulation. Alcohol evaporates and then it's gone with no lasting effect. Some pathogens are particularly sensitive or insensitive (or worse, develop resistance) to specific agents (especially because some agents are broad-spectrum and others are more mechanism-specific in their mode of action). Hard to answer the question specifically because "in some cases" is pretty vague...includes everything from playground scrapes to surgical incisions to cases of MRSA. DMacks (talk) 18:15, 14 February 2011 (UTC)
- It depends a bit on why you're using it. The modern version of good ol' fashioned iodine is Povidone. One important difference between the two is that if you where to use an iodine preparation on a daily basis, one would eventually absorb too much through the skin. However, for some indications it is certainly better. In the first world war, alternatives to iodine was garlic and cannabis --Aspro (talk) 20:21, 14 February 2011 (UTC)
- Neosporin also contains a skin protectant and the antibiotic in it is benign, it doesn't cause cell death. The AMA is no longer recommending you use alcohol or peroxide on wounds (not sure about iodine I know it's used in medicine still for example prior to catheterization in a female), the fact it kills cells means that it does more harm than good. 65.29.47.55 (talk) 22:31, 14 February 2011 (UTC)
Atomic structure
When one atom moved away from anywhere very time atomic level are not same but it more equilibrium than other for fixed position with some wave not particles very much to minimum ways series.Reaction position are not same means the wave are not similar for bad condition create some how. — Preceding unsigned comment added by Vowies (talk • contribs) 17:53, 14 February 2011 (UTC)
- I'm sorry, but don't think I understand you. Can you try writing that again using proper grammar and punctuation? Dauto (talk) 18:59, 14 February 2011 (UTC)
- Or, if English isn't your first language, please write in your first language and someone will translate. Or, see if there is a reference desk on the Wikipedia in your first language. --Tango (talk) 23:17, 14 February 2011 (UTC)
The Ever Eluding Question
Do plants feel pain?? Dutch scientist Marcel Dicke, of the Agricultural University in Wageningen, Holland, found evidence that all plants perform similar actions to the trees, when under threat from predators. Indeed, the level of sophistication in this process is made all the more remarkable by the fact that the these ‘signals’ encourage production of substances tailored to specific pests! An example of this would be the lima bean. When attacked by spider mites, the plant releases a chemical attractant for other types of mite, which prey on the attackers. Some plants help others, as in the case of cabbages, which release foul smelling isothiocyanates, discouraging aphids from attacking neighbouring plants like broad beans. —Preceding unsigned comment added by 1.23.10.106 (talk) 18:14, 14 February 2011 (UTC)
- I agree these are survival mechanisms and nowhere close to "animal" like senses but how to concisely refute the theory that plants feel pain in? —Preceding unsigned comment added by 1.23.10.106 (talk) 18:19, 14 February 2011 (UTC)
- Plants do not have pain nerves or a brain to receive signals from the pain nerves. To claim that plants feel pain will require defining what you mean by pain in such a way that it is not dependent on the biological process of receiving and processing signals from pain nerves. -- kainaw™ 18:36, 14 February 2011 (UTC)
- Nociception may be one such term - for example, Paramecium displays nociception, trying to back off and evade a fine needle.[5]
- One way in which Temple Grandin concludes that many (not sure about all) insects do not feel pain is that they will walk on broken limbs, which no vertebrate will do. Just putting that out there. From Animals in Translation. --Mr.98 (talk) 18:58, 14 February 2011 (UTC)
- Here's a related question: how do you concisely show that human beings (other than yourself) feel pain? It's entirely possible that you're the only sentient human, and that all other humans are robots without any consciousness. If you punch a human, how would you know whether that human is responding because he has the subjective experience of "pain", or because he's hard-wired to respond to harmful stimuli so as to minimize that stimuli.
- In this case, you might use induction to argue that because you feel pain, and because other humans have nearly identical biochemistry, organs, nervous systems, and reactions to stimuli, they probably feel pain too. However, you can't conclude from this that plants don't feel pain, because you can't prove that the mammalian body is the only system that can feel pain. In fact, it would be very surprising if this were true: why should this one path that evolution semi-randomly decided to take be the only one that leads to the subjective experience of pain? --99.237.234.245 (talk) 20:20, 14 February 2011 (UTC)
- Pain: the conscious, subjective experience that something unpleasant or harmful has happened. I think this definition is much closer to what people usually mean by "pain". Since the OP obviously isn't asking how to prove that plants have no nerves or brains, I assumed that he meant "pain" in the everyday sense of the word, and was not using the biological definition. --99.237.234.245 (talk) 23:03, 14 February 2011 (UTC)
- There is no evidence that a central nervous system is required to be conscious. The fact that humans have a central nervous system means only that the one evolutionary path Earth's animals happened to follow can lead to consciousness. It does not imply that this is the only path, or the most likely path, or the most common path. For all we know, sentient alien creatures might have an entirely chemical "nervous system", transmitting chemicals along structures similar to Earth plants' xylem and phloem. --99.237.234.245 (talk) 01:15, 15 February 2011 (UTC)
- Not to detract from the scientific interest of the question, but maybe you might ask for modern philosophy references from the humanities desk. The reason I suggest this is that beyond what we know about nervous systems and empirical science (and I think Temple Grandin's comment is an excellent empirical argument), there is an existential element of the question to which our backgrounds might not do justice. To that end, check out Bentham's animal rights philosophy, and this nice response with lots of links (but from an MA only, so take appropriate salt). And of course our paranormal plant perception article goes into the actual arguments a bit as well. SamuelRiv (talk) 00:03, 15 February 2011 (UTC)
- I've read that some trees produce a toxic substance when animals eat their leaves. This prompts the animals to move to the next tree. You could perhaps consider this as an algorithm executed by the tree to protect itself. Arguably, pain is the running of any type of algorithm that overrides/restricts normal function. Count Iblis (talk) 01:02, 15 February 2011 (UTC)
- You jostle a car and an alarm sounds. It is an algorithm, perhaps a basic sort of nociception, but does the car feel pain? In truth we have nothing resembling a theory of consciousness. We say that certain neural pathways carry pain because individual persons say they don't feel it when they are anaesthetized or severed. But how do you know even that what a person feels is only what they say? How do you know that the person who comes out of the operation in twilight sleep isn't saying he's alright, but somewhere "unconsciously" he has been in agony? Can you generalize further to say that people are the only consciousness, and no other pathway can feel pain? I don't see a scientific basis to disprove that the clouds feel pain when an airplane flies through them. Wnt (talk) 15:22, 15 February 2011 (UTC)
- That is the problem here. In order to claim that plants feel pain, the definition of pain is being generalized to purposely include plants. The side-effect of that is that other things (like cars) get included in the generalized definition. So, it is not a discussion about feeling pain. It is a semantic argument about how to purposely include plants without including other things - which is not in any way science. -- kainaw™ 15:26, 15 February 2011 (UTC)
- I agree, but then one can always assume that a conscious entity is identified with an algorithm that is running. This is equivalent to the strong AI hypothesis. So, Wnt is generated whenever we run that particular program that is currently implemented by the neural network that his brain is running. Any entity that is experiencing pain clearly has its normal algorithm being interfered with by the running of an emergency algorithm. In case of a car, you have to assume that without the alarm going off, there is an algorithm running and from the perspective of that algorithm, the alarm going off, is restricting things in some way. Of course, this approach can be criticised as being not very scientific. However, it may be that deep down all that exists are algorithms, i.e. that all that exists is simply a mathematical multiverse. It then makes sense to talk about the world that any given algorithm subjectively perceives as a mathematical representation of the algorithm. Count Iblis (talk)
You are touching on the philosophical question of "Other Minds." There has a rich tradition going back at least to Descartes. A good introduction is the Brain in a vat article. —Preceding unsigned comment added by 12.186.80.1 (talk) 16:19, 15 February 2011 (UTC)
Time dilation measured by radioactive decay
I have a question concerning my better understanding about time dilation. I am wnadering if it is just mathematic aspects or it does really have physical meaning.
Supose the experiment with two observers. One in land, stationary with no movement. The other is travelling inside one rocket with speed around 0.8 light speed (c). If they have the same amount of radioactive matter, uranium or others. What we would observe when the rocket return to start point after one travel that had taken 01 thousand years. Would they see different carbon contents in these two samples or it will be the same. My question is based in the fact that time dilation always has been explained using the time interval between events. This trial has different concept.
Obs: I am not english native. — Preceding unsigned comment added by Futurengineer (talk • contribs) 20:18, 14 February 2011 (UTC)
- This experiment doesn't have a different concept. If the time interval between individual particle decays dilates, the half-life must get longer, because the sample is now decaying more slowly. If you put uranium on a spaceship and make it travel at high speed, the spaceship sample will decay more slowly as seen from Earth than an Earth-bound sample, by a factor of gamma=1.67. --99.237.234.245 (talk) 20:36, 14 February 2011 (UTC)
For example, in introductory physics, a common homework problem requires application of relativistic time dilation to explain the arrival rate of muons originating from cosmic rays. These muons decay too quickly, and they have no business arriving at Earth's surface as frequently as they do; but because they are traveling at relativistic speeds, the decay half-life measured in the laboratory is not the same as the decay half-life measured in the co-moving frame of the muon. A model of cosmic ray production in the upper atmosphere, from SLAC, works the math properly for you, and explains the physics of muon production as a result of cosmic hydrogen ions colliding in the thermosphere. Nimur (talk) 23:25, 14 February 2011 (UTC)
After reading this article, I still have no idea how it's supposed to get fusion to occur. Is the idea to get hot plasma as dense as possible and loiter it around each other until the particles can quantum tunnel through and produce fusion? ScienceApe (talk) 21:23, 14 February 2011 (UTC)
- Fusion is actually the trivial part: heat hydrogen enough and it just happens. The hard part is making it continue to happen: as soon as your fusing plasma touches any normal-temperature material, its heat is sapped away and the reaction stops; similarly if it spreads out too much. Thus "confinement"; magnetic confinement is just the clever application of magnetic fields to hold the fusing plasma in place for a "sufficient" time to be interesting/productive. --Tardis (talk) 21:40, 14 February 2011 (UTC)
- Couple of questions. How do they heat it in magnetic confinement? I know they use lasers in inertial confinement (usually). Why is the torus shape preferable over a spherical shape? Would this be easier to do in a zero gravity environment like outerspace? ScienceApe (talk) 21:45, 14 February 2011 (UTC)
- I think it's usually RF heating: the plasma is conductive and can receive energy like an antenna. Torii are good because the magnetic field is solenoidal: the field lines have to form loops, so it makes sense to make the device a loop as well. I don't think gravity is a problem: one ought to be able to counteract it with a static electric field (and a slightly charged plasma). --Tardis (talk) 21:54, 14 February 2011 (UTC)
- Couple of questions. How do they heat it in magnetic confinement? I know they use lasers in inertial confinement (usually). Why is the torus shape preferable over a spherical shape? Would this be easier to do in a zero gravity environment like outerspace? ScienceApe (talk) 21:45, 14 February 2011 (UTC)
Watson the computer system
Would the Watson computer system likely be capable of passing the Turing test? Googlemeister (talk) 21:46, 14 February 2011 (UTC)
- (EC) Presuming you mean Watson (artificial intelligence software), that seems rather unlikely. Amongst other things, if it could you would think IBM would be making more noise about that rather then hyping its Jeopardy! skills... Nil Einne (talk) 22:05, 14 February 2011 (UTC)
- IBM isn't even claiming that their system is "turing-test ready." They're simply advertising that it has sophisticated capability for answering questions (or ... questioning answers) posed in natural language format. (I'll also comment that those East-Coast intellectuals seem to have designed a nerdy operating system. Out here in California, our robots are free-range outdoorsy-types who enjoy hiking and long walks on the beach... not crashing out in front of the TV watching Jeopardy. Nimur (talk) 22:33, 14 February 2011 (UTC)
- The article you linked has a section on the [Prize], for which "the first contest was won by a mindless program with no identifiable intelligence that managed to fool naive interrogators into making the wrong identification," which is particularly evident in Turing competitions where they have ordinary citizens as judges. But for some perspective on what the Turing Test actually means in terms of consciousness, maybe check out both recent and early transcripts of the Loebner competitions. For all of Turing's genius, his test appears to be short-sighted - perhaps we should start thinking about an improved version video? SamuelRiv (talk) 00:39, 15 February 2011 (UTC)
- There is no "official" Turing Test, so the question is unfortunately a little too vague. The Loebner Prize above has tried to make itself into the "official" test, but it's not a test that any scientists participate in, only chat-bot writers with lots of time on their hands. (And they are still pretty terrible -- here's the transcript from the 2009 winner. Really no different from the early Eliza programs.)
- One answer might be "Does Watson play Jeopardy in a human-like manner?" That is, might you think a human was giving those answers? If so, Watson has passed a very limited Turing Test. Watching the game last night, the answers were very good, unlike a few months ago when the answers didn't match the questions. So it may have passed this Turing Test. In terms of conversational ability, however, it's not even trying. Therefore, it wouldn't pass the Turing Test as normally envisioned. — Sam 63.138.152.135 (talk) 14:43, 15 February 2011 (UTC)
There is already a method which can answer questions given sufficient information in the query and the database. An example would be if you asked, "What country is known by a pattern of 13 alternating red and white stripes with 50 white stars on a blue background in the upper left hand corner?" or similar question. (this example) Notice that an item (flag in this case) can be identified with far less information after it is classified and then the database optimised. --Inning (talk) 17:43, 15 February 2011 (UTC)
February 15
California power plant's NOx emissions
I have had a good look around and have been unable to find an answer for this. I was wondering if anyone else had anymore luck finding the regulated NOx emissions limit (in ppm or otherwise) for power generating plants in California? Any help would be appreciated. Cheers 150.49.180.199 (talk) 02:50, 15 February 2011 (UTC)
Decompressing the spine
Is there a reason that some people who have had a back injury feel their spine get compressed throughout the day and feel better when they decompress it with an inversion table and people who have not had a back injury do not feel like their spine is getting compressed? —Preceding unsigned comment added by 76.169.33.234 (talk) 06:51, 15 February 2011 (UTC)
- That's right: one doesn't need to have had a back injury to feel the results of the compression (OR). Dbfirs 08:15, 15 February 2011 (UTC)
SPEED OF LIGHT
I am a student of science and want to know that light has a speed of 3*10^8 m/s. Why not greater than it?Mohammad babar (talk) 07:38, 15 February 2011 (UTC)
- http://en.wikipedia.org/wiki/Meter#Speed_of_light 157.193.175.207 (talk) 08:13, 15 February 2011 (UTC)
- That (the definition of the meter in terms of the speed of light) is of course a very recent definition, and both the meter and measurements of the speed of light existed before that definition. OP's question makes more sense if turned around: Given the speed of light (and the values of the other constants of nature), why do atoms and people have the sizes that they have? That is a question that can actually be answered. The values of the constants of nature are contingent (at least in the context of our present theories), we do not know of any reason why they are what they are. --Wrongfilter (talk) 09:14, 15 February 2011 (UTC)
- This page claims that if the speed of light was higher, nuclear decay would be faster and nuclear reactions in stars would be faster. Therefore the sun might have gone out by now, even if it hadn't the Earth would be much colder (because it gets some of its heat from nuclear decay) and the universe would last a much shorter period of time. Possibly this would be too little time for human beings to evolve - see anthropic principle - but that is just my speculation. --Colapeninsula (talk) 10:13, 15 February 2011 (UTC)
- This is a valid argument that the values are such as they are (inferred from the observation that we exist). It is (scientifically) not acceptable as an argument why they are such as they are. --Wrongfilter (talk) 11:26, 15 February 2011 (UTC)
- Actually, that is debatable. See anthropic principle. If the constants were significantly different, we wouldn't be here to observe them. Or, in other words, the fact that the universe can sustain life is a precondition for us being able to observe it. --Stephan Schulz (talk) 12:05, 15 February 2011 (UTC)
- Which is exactly what I'm saying. From the observation of our existence we can infer what the values are, because if they were different we wouldn't be here. But we cannot claim that our existence is the reason or the cause (in some teleological sense) for their existence. They were not chosen such that we might come into existence. --Wrongfilter (talk) 12:52, 15 February 2011 (UTC)
- Actually, that is debatable. See anthropic principle. If the constants were significantly different, we wouldn't be here to observe them. Or, in other words, the fact that the universe can sustain life is a precondition for us being able to observe it. --Stephan Schulz (talk) 12:05, 15 February 2011 (UTC)
- This is a valid argument that the values are such as they are (inferred from the observation that we exist). It is (scientifically) not acceptable as an argument why they are such as they are. --Wrongfilter (talk) 11:26, 15 February 2011 (UTC)
- Is Mohammad actually seeking an explanation of why light has a maximum speed? You may find the article Introduction to special relativity useful, or maybe the Simple English Wikipedia article, which you can read by clicking here. If you tell us a language you prefer, we can show you explanations in that language. 86.164.25.178 (talk) 11:58, 15 February 2011 (UTC)
You can always ask that question though. If the speed of light was 100 mph faster, you could then ask why can't it be faster than that? If the question is, why is the speed of light what it is, I'm not sure we have an explanation of why it goes at that speed. That's just what we observe its speed to be. Obviously, reading about Special Relativity should answer some other questions. We do know that light travels slower through a medium like air or water. Also the speed of light may have been different in the past, maybe someone can expound on that. ScienceApe (talk) 15:09, 15 February 2011 (UTC)
- On the latter point, see Variable speed of light. There is not a lot of compelling reasons to go with a VSL theory at this point. As for why the constants are what they are, nobody is really sure. There are different ways to approach the question. The anthropic principle brought up earlier says that if they weren't what they were, we wouldn't be able to observe them, thus we don't have much of a place for suggesting that they could be different. Those who believe in the possibility that this is just one of many universes that pop in and out of existence regularly (see multiverse) see it as something like natural selection (only some universes are going to stay stable, only some produce life, and here we are, in one of those). (On all of these points, see Fine-tuned Universe.) Some suggest that the exact answer why might be revealed by further work in string theory cosmology; that it will simply be logically impossible for it to be any other way. (It's not clear that this will happen, though; a lot of string theory cosmologies say that you could, in fact, have all sorts of different constants.) Personally I think this sort of thing gets well into metaphysics, even when scientists are doing it, and that there isn't a lot of evidence one way or the other at this point. If you want to say, "God did it," as some clearly do, that's an answer, though in the past we've found that it's generally not as satisfying an answer than the naturalistic ones (and we answer a lot fewer questions with "God did it" these days than we used to). --Mr.98 (talk) 20:38, 15 February 2011 (UTC)
- Saying "god did it" isn't even an answer, it's an Argument from ignorance. ScienceApe (talk) 02:45, 16 February 2011 (UTC)
- Not necessarily. It depends on one's definition of "God". ←Baseball Bugs What's up, Doc? carrots→ 21:43, 16 February 2011 (UTC)
- Yeah, you're the guy who once said atheism is a religion. Saying "god did it" is an argument from ignorance unless your "definition of god" is the scientific theories proposed which explain observed phenomena, in which case I have no idea why you are calling it god. ScienceApe (talk) 14:40, 17 February 2011 (UTC)
- But atheism is a religion. Atheists still believe that the universe has laws (not just scientific laws, but also laws without clear scientific basis (for example) explaining whether an unborn fetus or a dog or an earthworm can feel meaningful pain). They still have some cosmogeny in mind, as to how scientific laws came to be as they are, even if it is by accident or anthropic principle. They believe in morals, in things that should be done or shouldn't. They have a conception of self, whether they feel it is limited to a span of spacetime or find some way to envision their atoms or genes or algorithm of consciousness continuing to be relevant in some other setting. All the basics of religion are there, except for the most colorful and allegorical traditions which personify God in a notably visual or tangible way. Wnt (talk) 07:17, 19 February 2011 (UTC)
- Yeah, you're the guy who once said atheism is a religion. Saying "god did it" is an argument from ignorance unless your "definition of god" is the scientific theories proposed which explain observed phenomena, in which case I have no idea why you are calling it god. ScienceApe (talk) 14:40, 17 February 2011 (UTC)
- Not necessarily. It depends on one's definition of "God". ←Baseball Bugs What's up, Doc? carrots→ 21:43, 16 February 2011 (UTC)
- Saying "god did it" isn't even an answer, it's an Argument from ignorance. ScienceApe (talk) 02:45, 16 February 2011 (UTC)
First thing, A religion can be defined as a set of beliefs, so atheism/agnosticism can be defined as a religion. Second thing, the speed of light is basically an axiom of modern physics, and there's no way to answer a question "Why is it so?". It's like asking "Why is 1+1 2?", or, "Why do an infinity of lines pass through a given point?". The value of the speed of light can be similarly explained (or with the anthropic principle). These problems aren't there only with the speed of light, though. Other constants like Planck energy, Planck time, and Planck length are just formed by combining G, h, and c in various ways, using dimensional analysis. These have a physical meaning, but you can't derive the values from the physical meaning. If you look at the derivation, there's no quantum mechanics involved. They've just found a way of combining the three constants to get a constant with the required dimensions. All these constants sound cobbled-together, but they work. Same thing with the speed of light.
If you want to go into the Standard Model, the speed of light is mathematically derived (not sure on this one). With M-theory, the speed of light is no longer the universal constant, but I'm quite sure it is mathematically derived there, too. ManishEarthTalk • Stalk 16:27, 19 February 2011 (UTC)
- Atheism is NOT a religion, it's a stance on a single issue. Atheism just means you don't believe in god. That's not a religion. If I don't believe in unicorns, is that a religion? If I don't believe in the Loch Ness Monster, is that a religion? The proposal that not believing in something, is a religion, is absurd to say the least. ScienceApe (talk) 16:37, 19 February 2011 (UTC)
somthing about the creation theory of earth
I know that this is not the place to say my own ideas but I ask an opportunity for saying about only this subject that refers to earth. excuse me . I want to know can i do so? Akbar mohammadzade Iran
This is the first time that my own idea is publishing and for the reason of what I think about it is my patent ,may be occure some difficulties in understanding that subject and i have to do so . I am trying to publish my theory .when it happen, I will say what I am thinking about the solar system specially about the earth . According to the data bases collected and the reality observations in astronomy and geology ,it is clear that the earth , mars , venous and mercury which known rock planets created several years after the creation of sun and gas giant planets :Jupiter , Uranus ,Saturn and Neptun. the last bodies of solar system are moons ,Pluto and sates , comas and…. Which has less age than earth . I am trying to upgrade recent astrophysics theories that they are approaching the solution of the paradoxes in theory and practice .--78.38.28.3 (talk) 09:16, 15 February 2011 (UTC)iran feb 2011 mohammadzade
- Sooooo what's your question? Someguy1221 (talk) 09:47, 15 February 2011 (UTC)
- He asks whether we mind if he tells us his new theory. And I think our advice is probably that he should find an internet forum where people discuss such things, rather than explain it here. Itsmejudith (talk) 16:37, 15 February 2011 (UTC)
- Reading Formation and evolution of the Solar System may help you. 92.15.16.146 (talk) 11:22, 19 February 2011 (UTC)
Tidal Force
I'm confused why the moon causes a high tide on the opposite side of the earth. The article 'Tidal Force' shows the tidal forces in figures 2 and 4. Could someone explain what "the residual force after the field of the sphere is deducted" means and why it results in a net outward force on the opposite side of the satelite? Thank you80.168.88.74 (talk) 11:47, 15 February 2011 (UTC)
- In short, on the side near to the moon, the moon pulls the water harder than the Earth in total (since it's about 6000 km closer than the center of mass of the Earth). On side opposite the moon, the moon pulls the Earth harder than the water (since the water is about 6000 km farther away than the center of mass). Think about it not as "the water is pulled somewhere", think " the Earth is pulled somewhere, the water remains". --Stephan Schulz (talk) 12:02, 15 February 2011 (UTC)
- In effect, the Earth is falling towards the Moon, at a speed that it wants to "on average". The parts of Earth nearest to the Moon want to fall faster than this average, so they're tugged away from Earth towards the Moon. The parts furthest from the Moon want to fall more slowly than this average, so they lag begind. 86.181.174.29 (talk) 12:36, 15 February 2011 (UTC)
- And this might be clearly not obvious, as we all should know that when objects fall on Earth, it doesn't matter how massive they are - they always fall with the same acceleration. However, this is only because the effective distances from the center (of mass) of the Earth to the objects that we throw in the air are about the same - as the poster above points out, the oceans on each side of the Earth are about 6000km away from the Earth's center of mass, which ends up making a "big" difference.
- I put "big" in quotation marks because remember that relative to the 6000km radius of the Earth, a tide of 1 meter is an utterly-miniscule astrophysical effect, unnoticeable if it were watched from another planet. SamuelRiv (talk) 16:34, 15 February 2011 (UTC)
Quark + Gluon plasma - Evidences
Reading article about matter states I could see reference about quark-gluon plasma that was found in CERN in 2000. From other books I have read that it wasn´t possible until now to detect any quark isolated. The evidence that they exist was taken by electrical effects when electrons travel close to the protons and was detected three concentrated points that have strong effect. It was assumed that they were 02 quarks up and 01 down. So is there more evidence in CERN 2000 experiment that confirms that quarks and gluons really exists ? — Preceding unsigned comment added by Futurengineer (talk • contribs) 11:58, 15 February 2011 (UTC)
- The evidence that quarks exist is as strong (at this point) as the evidence that electrons exist. No one has ever seen either one. The evidence comes from the success of the electron/quark picture at predicting the results of all kinds of experiments, and the failure of every alternative model to do the same. It's not especially important to observe the particles "isolated" (though that does tend to rule out alternative theories). The quarks in a quark-gluon plasma are surrounded by other quarks, so they aren't really isolated. For that matter, an isolated electron is undetectable too; they have to interact with surrounding matter to be "seen". -- BenRG (talk) 22:29, 15 February 2011 (UTC)
Schwarzschild Metric
If one sets the angular terms to be zero (by considering a particle travelling on a radial line of the field source) and also sets the spacetime interval to be zero (by considering a photon), the equation for dr/dt does not give c. Why? —Preceding unsigned comment added by 129.67.37.227 (talk) 13:43, 15 February 2011 (UTC)
- In general relativity the coordinates are arbitrary and meaningless except as interpreted by the metric. So the t coordinate is not true time, nor is the r coordinate a true distance. They are just numbers that label events in the spacetime, and in order to find distances and times you need to use the metric.
- Alternatively, find a local coordinate transformation that makes the metric into the diag(1,1,1,-1) of SR. Then in that frame your photon will move with speed c (tautologically because you selected it to have null worldline). –Henning Makholm (talk) 14:22, 15 February 2011 (UTC)
Discontinuing a vaccine
Isn't it always to risky to stop immunizing your population against a well-known illness? Considering that there are reclusive states which could be, intending or not, a source of a new outbreak. Quest09 (talk) 15:37, 15 February 2011 (UTC)
- In a practical sense, consider the case of smallpox. It is basically eradicated from the wild, however it still exists in laboratories, and could be weaponized by parties with ill intent. If you were a policymaker, you'd have to balance the risk of smallpox being used as an offensive weapon against the risks inherent to the vaccine. Smallpox vaccines have a rate of complications/infections of something like 14-500 per million according to our article. So in a country of 300 million people, that is a fairly large "peacetime" number of people suffering (it would be a justified number compared to that of a smallpox outbreak when it was still "wild"). Whether or not that is a good bet with regards to the threat of smallpox as an agent of war or terrorism is unclear; the US has evidently decided it is not, and does not vaccinate against it generally. Note that in the case of a weaponized threat, you can also say that you might have non-vaccination means of prevention, e.g. a state of deterrence with other nations, or vigorous counter-terrorism strategies. Each of these approaches have their own risk levels and possible numbers of incidental deaths (e.g. you will have a certain number of false positives with your counter-terrorism strategies that may lead to collateral deaths, infringements of liberties, etc.). In all of these situations, people have to make judgments about relative risks and balance accordingly. --Mr.98 (talk) 17:42, 15 February 2011 (UTC)
- We need not limit ourselves to the one human disease that's been eradicated either. Take Yellow fever: there exists a vaccine that is largely effective and fairly safe. It's not 100% safe and (1 severe reaction for every 200,000 to 300,000 people, according to the article), perhaps more importantly, it's not free. For the vast majority of people living in the United States and Europe, the chances of contracting yellow fever are slim to none, and the vaccine's not worth it (or at least, the CDC and similar bodies in unaffected areas feel it isn't worth it for most people [6]). Buddy431 (talk) 03:48, 16 February 2011 (UTC)
- In a practical sense, consider the case of smallpox. It is basically eradicated from the wild, however it still exists in laboratories, and could be weaponized by parties with ill intent. If you were a policymaker, you'd have to balance the risk of smallpox being used as an offensive weapon against the risks inherent to the vaccine. Smallpox vaccines have a rate of complications/infections of something like 14-500 per million according to our article. So in a country of 300 million people, that is a fairly large "peacetime" number of people suffering (it would be a justified number compared to that of a smallpox outbreak when it was still "wild"). Whether or not that is a good bet with regards to the threat of smallpox as an agent of war or terrorism is unclear; the US has evidently decided it is not, and does not vaccinate against it generally. Note that in the case of a weaponized threat, you can also say that you might have non-vaccination means of prevention, e.g. a state of deterrence with other nations, or vigorous counter-terrorism strategies. Each of these approaches have their own risk levels and possible numbers of incidental deaths (e.g. you will have a certain number of false positives with your counter-terrorism strategies that may lead to collateral deaths, infringements of liberties, etc.). In all of these situations, people have to make judgments about relative risks and balance accordingly. --Mr.98 (talk) 17:42, 15 February 2011 (UTC)
- Yes, see also herd immunity and anti-vaccine conspiracy theories. There has been opposition to vaccines by some, ever since they have been invented. And there have been actual cases of actual problems with vaccines. As a general rule, though, the anti-vaccination crowd is full of nutters, motivation by religion or philosophy, not by the weight of evidence. Friday (talk) 17:03, 15 February 2011 (UTC)
- Some opposition may be due to Unethical human experimentation in the United States. Smallman12q (talk) 23:50, 15 February 2011 (UTC)
- No one has mentioned the more resent example of BCG. This is another vaccine whose wide spread use is being curtailed and focused instead on high risk groups. The main reasons are: First it is no longer cost effective. The number needed to treat are now about 10,000 vaccinations to prevent a single case. “Secondly the harm done in adverse effects from the vaccine, usually abscesses at the sight of injection, outweigh the preventive effect.” Also, it is not very effective in some groups. Here is a fairly clear article about the UK position on BCG. Frequently Asked Questions about BCG. It remains however, a relitivly safe vaccine as you can see from the Complications of bacille Calmette-Guérin (BCG) vaccination and immunotherapy and their management.--Aspro (talk) 10:50, 16 February 2011 (UTC)
Chemical equation
alkyne (CnH2n-2) + Hydrogen sulfide (H2S) = ???????????????????? RahulText me 17:19, 15 February 2011 (UTC)
- Not knowing if it actually reacts that simply, but our article on alkynes says that the analogous reaction between an alkyne and water yields an enol which tautomerizes to an aldehyde or ketone. <OR> So if it reacts I'd guess that it forms either an aldehyde or ketone (depending on where in the alkyne the triple bond was) in which the oxygen is replaced by a sulfur atom</OR>. 178.26.171.11 (talk) 20:59, 15 February 2011 (UTC)
- I can't find any cases of addition to alkynes by the same reaction methodology as for water (reaction begins with alkyne getting protonated to give cationic alkene, then neutral water attacks), but rather I see examples under nucleophilic conditions (looks like sulfur anion attacks alkyne). I see lots of examples using RSH rather than HSH as OP asks, and these give the vinyl thioether (obviously cannot tautomerize to the thioketone). Looking specifically for thioketone products of alkyne reactions though, I'm not seeing many. I do see articles commenting that the ene-thiol is much less unstable than the comparable enol and that the energy barrier to the tautomerization is fairly high...interesting! DMacks (talk) 21:38, 15 February 2011 (UTC)
- Hydrogen sulfide seems to be a common contaminant in acetylene, so that suggests that they do not react under pressure at room temperature. However I did find this: http://www.springerlink.com/content/lh51828q5101k712/ or http://elibrary.ru/item.asp?id=12690943 "QUANTUM-CHEMICAL INVESTIGATION OF THE MECHANISMS OF REACTIONS INVOLVING NUCLEOPHILIC ADDITION TO ACETYLENE. 3. MECHANISM OF THE FORMATION OF VINYLTHIO ANIONS" by N.M. Vitkovskaya, O.Yu. Dolgunicheva, F.S. Dubnikova, B.A. Trofimov where divinyl sulfide is formed in the presence of KOH in dimethylsulfoxide. Graeme Bartlett (talk) 09:44, 17 February 2011 (UTC)
- I can't find any cases of addition to alkynes by the same reaction methodology as for water (reaction begins with alkyne getting protonated to give cationic alkene, then neutral water attacks), but rather I see examples under nucleophilic conditions (looks like sulfur anion attacks alkyne). I see lots of examples using RSH rather than HSH as OP asks, and these give the vinyl thioether (obviously cannot tautomerize to the thioketone). Looking specifically for thioketone products of alkyne reactions though, I'm not seeing many. I do see articles commenting that the ene-thiol is much less unstable than the comparable enol and that the energy barrier to the tautomerization is fairly high...interesting! DMacks (talk) 21:38, 15 February 2011 (UTC)
Immunology and parasites
There's the whole hygiene hypothesis that claims in short that "lack of contact with parasites makes our immune systems nonfunctional." This is usually phrased as the immune system lacks appropriate targets and therefore takes pot shots at things it's not supposed to attack. Has there ever been any serious evaluation of whether the parasites have an immunomodulatory effect, essentially "turning down" the immune system to help their own survival? This would make sense from a coevolution standpoint (i.e. the immune system developed as overreactive because it "assumed" it would be weakened by parasites), but is there any evidence of this? SDY (talk) 20:50, 15 February 2011 (UTC)
- There is an article on Helminthic therapy if thats any help.--Aspro (talk) 21:45, 15 February 2011 (UTC)
- Of course AIDS turns down the immune system, as its very name implies. There aren't many other parasitic or infectious agents that do, though. See immunodeficiency for more information (but not much).Looie496 (talk) 21:55, 15 February 2011 (UTC)
- Well, I don't know about many either, but two common viruses that do, are those responsible for Chicken Pox and Measles. Both have suppressant effects on the immune system and so complications can come in the form of secondary infections. This is why it is important not to vaccinate against these, with live viruses, whilst the immune system is suppressed due to other reasons. --Aspro (talk) 17:35, 16 February 2011 (UTC)
- Of course AIDS turns down the immune system, as its very name implies. There aren't many other parasitic or infectious agents that do, though. See immunodeficiency for more information (but not much).Looie496 (talk) 21:55, 15 February 2011 (UTC)
Guadalquivir - second or fifth longest river in Spain?
I've already posted this question in the discussion about the article but nobody has answered...So here it goes again:
In the German article it says the Guadalquivir is the fifth longest river in Spain (after Tagus, Ebro, Douro and Guadiana). I don't know how you would count rivers that also lie in Portugal, but at least for Ebro, which lies entirely in Spain, you just have to compare the lengths in the English articles (Ebro 910 km vs. Guadalquivir 657 km) to see that something is wrong with the statement "The Guadalquivir is the second longest river in Spain (after Tagus)". So which rank does the Guadalquivir actually have? —Preceding unsigned comment added by 77.0.237.4 (talk) 20:57, 15 February 2011 (UTC)
- The Spanish WP has quinto río por longitud. Oddly enough, they state the length is 722 km, whilst the en & de WP has 657 km. It must be a consequence of the rain in Spain... --Cookatoo.ergo.ZooM (talk) 21:47, 15 February 2011 (UTC)
Chemical thermodynamics vs. Thermochemistry
Could someone give an explanation of the main difference between Chemical thermodynamics and Thermochemistry, I've read the articles but haven't found a good explanation. /Natox (talk) 21:30, 15 February 2011 (UTC)
- The differences are a bit fuzzy, that is the boundaries between the two topics overlap a lot. One textbook I teach from (Brown, LeMay, Bursten The Central Science) roughly considers topics such as calorimetry and specific heat to be squarely in the realm of thermochemistry and free energy and entropy to be firmly in the realm of chemical thermodynamics, but honestly there's not a lot of fundemental difference between the two topics. It would seem that they treat thermochemistry as dealing more with heat transfer between substances, while they treat chemical thermodynamics as energy changes (including enthalpy and entropy and free energy) that occur during chemical reactions. However, one could clearly see how those topics could easily overlap. --Jayron32 01:02, 16 February 2011 (UTC)
Black holes
This may sound like a really stupid question... When a guy approaches the event horizon of a black hole an outside observer will see his clock slowing down, and that he will never actually reach the event horizon (because time stops there). But they also say the guy falling in will reach the center of the hole in finite time, so as he crosses the event horizon he will see time outside speed up infinitely and past eternity!? I wonder what would the universe look like after an infinite amount of time. Money is tight (talk) 22:43, 15 February 2011 (UTC)
- We have an article on that. Check out Ultimate fate of the universe. TenOfAllTrades(talk) 22:58, 15 February 2011 (UTC)
- Yes, that is correct. The universe would be so distorted by gravitational lensing effects and so blue-shifted that you wouldn't actually be able to see much, though. You would get infinite blue-shift by the time the universe you were viewing was infinitely old, so you wouldn't see anything at all. --Tango (talk) 23:01, 15 February 2011 (UTC)
- No, an infalling observer does not get to see the end of the universe. Some of the light he emits immediately before crossing the horizon will take so long to get out that there'll always be some of it left close to the hole, but that does not mean that light from an arbitrary event outside the hole has time to catch up with the observer before he hits the singularity. (The infalling light is subject to the same apparent slowdown as a massive astronaut, so it's hard for it to catch up). –Henning Makholm (talk) 01:48, 16 February 2011 (UTC)
- Since there are two contradictory answers above I will weigh in. Makholm is right. The falling observer can only see events inside his past light cone and that does not include the end of the universe. The idea that if A sees B slow down to a halt than B must see A speed up to infinite speed is just wrong. See for instance the situation where two observers are moving relative to each other. They BOTH see the other observer slow down. Dauto (talk) 03:55, 16 February 2011 (UTC)
- The infalling observer will also see the Hawking radiation from the event horizon get blue shifted. Just because radiation is blueshifted, does not make it invisible. A thermal blackbody spectrum blue shifted will look like a hotter, and brighter blackboddy spectrum, so the universe will not become invisible to these observers, but should look brighter and brighter over time. The time experienced for this will also get shorter and shorter, so the period for a gamma ray blast may be apparently short, but with an infinite period of light and radiation from the universe outside coming in, the energy should also be infinite, and any infalling object should be expected to be destroyed before meeting the event horizon.Graeme Bartlett (talk) 10:24, 16 February 2011 (UTC)
- Actually, you don't see any Hawking radiation if you freefall through the event horizon; you only see it if you accelerate to stay outside. (Yes, that means that some people detect particles where other people detect none—see Unruh radiation.) -- BenRG (talk) 20:09, 16 February 2011 (UTC)
- I agree with Henning Makholm and Dauto - the infalling observer does not see the end of the universe, neither are they destroyed by a burst of blue-shifted gamm rays. As Dauto says, these ideas assumes a symmetry between infalling observer and distant observers that simply does not apply. Our event horizon article says "An observer crossing a black hole event horizon can calculate the moment they've crossed it, but will not actually see or feel anything special happen at that moment". As long as they are small and tough enough or the black hole is large enough for them to survive spaghettification for a sufficiently long time, then they can cross the event horizon intact. Gandalf61 (talk) 10:59, 16 February 2011 (UTC)
- I agree with Henning Makholm, Dauto and Gandalf61. I think perhaps others are confusing the perspective of an infalling observer with the perspective of an observer that's just barely outside the event horizon, accelerating like crazy in order to remain at a constant Schwarzschild r coordinate. Arbitrarily close to the event horizon, such an observer would indeed in principle see an arbitrarily blue-shifted, arbitrarily sped-up version of the outside universe. Of course, the thrust required to maintain a constant r coordinate also approaches infinity as the distance to the event horizon approaches zero, so there's a practical limit as to how much of the future of the universe you can actually see that way. A human observer could only actually see a negligible amount of speeding up of the outside universe that way at an outward acceleration small enough to keep from turning into a puddle of red goo. Red Act (talk) 12:37, 16 February 2011 (UTC)
- In further agreement, let's just remember that seeing "infinitely blueshifted light" means travelling at the speed of light. But even inside a black hole, no one travels faster than light relative to the curved spacetime present there. And if you don't see infinitely blueshifted light, that means that when you look back at a source producing any given frequency of light, you can (at least in concept) count the number of individual wave oscillations that reach you - which means that you're seeing individual ticks of a clock, so infinite time never appears to pass. Wnt (talk) 18:45, 16 February 2011 (UTC)
- Would the blueshift be visibly apparent before spaghettification killed the man? Googlemeister (talk) 19:28, 16 February 2011 (UTC)
- Whether there's redshifting or blueshifting going on, by the way, depends on which direction the infalling observer looks in. As per Gullstrand–Painlevé coordinates#A rain observer's view of the universe, as of when the infalling observer crosses the event horizon, at least, the stars that are behind the observer are redshifted, and the stars visible around the edge of the black hole are blueshifted.
- The answer to your question is that it depends on how big the black hole is. Assuming the infalling observer is freefalling from rest at infinity (or from a matching velocity and position), the amount of redshifting and blueshifting the observer sees at a given time depends purely on how many Schwarzschild radii the observer is from the black hole. But how many Schwarzschild radii you can get from a black hole without getting spagettified depends on the size of the black hole. With a supermassive black hole, you can get well inside the event horizon without getting spaghettified. And the redshifting and blueshifting would be quite noticeable by the time you reach the event horizon, so you would be able to see those effects in that case. But with a smaller black hole, the tidal force on a human as of a given number of Schwarzschild radii is greater, because the ratio of the human's size to the Schwarzschild radius is larger, so you might not be able to notice any redshifting and blueshifting before you get spaghettified if the black hole is too small. Red Act (talk) 22:07, 16 February 2011 (UTC)
- Would the blueshift be visibly apparent before spaghettification killed the man? Googlemeister (talk) 19:28, 16 February 2011 (UTC)
- In further agreement, let's just remember that seeing "infinitely blueshifted light" means travelling at the speed of light. But even inside a black hole, no one travels faster than light relative to the curved spacetime present there. And if you don't see infinitely blueshifted light, that means that when you look back at a source producing any given frequency of light, you can (at least in concept) count the number of individual wave oscillations that reach you - which means that you're seeing individual ticks of a clock, so infinite time never appears to pass. Wnt (talk) 18:45, 16 February 2011 (UTC)
Science and Miracles
This is a two parts question. First, can we classify frankly scientific miracles as pseudoscience? I can see here some articles discussing scientific miracles from the cultural point of view but not categorizing them in the pseudoscience area even though they look very similar.
The second part is not directly related to Wikipedia questions, but I am thinking of writing a book or even an article treating this part in details and was wondering if there were some volunteers to share the idea. I can collect enough materials related to Qur'an and miracles subject but I will lack for the miracles believed in other religions and cultures. I believe it would be interesting.--Email4mobile (talk) 23:17, 15 February 2011 (UTC)
- We don't have an article on scientific miracles — I see from Googling that it is the practice of trying to find references in scripture to things that would not have been able to be known at the time, which would give credence to the idea that the scripture in question was of divine origins. (E.g. the equivalent of finding a great description of a Boeing 747 in the Bible somewhere.) (Note: our article on this is apparently at Scientific foreknowledge in sacred texts). Those that I have seen, primarily relating to Islam, have been fairly ridiculous — very bizarre interpretations of very vague passages and then trying to claim that these very poetical statements are really evidence of knowledge of very specific scientific theories. This approach also serves to cherry pick quite liberally — things that are apparently scientifically inaccurate (e.g. quite straightforward descriptions of the Sun as a satellite of the Earth) get thrown out or interpreted away. It's definitely not a scientific approach; it's inherently unfalsifiable, and involves utilizing a number of logical fallacies. Whether it is pseudoscience depends on whether the practitioners pretend it is scientific or not. Just because something is being offered as evidence does not mean it has pretensions to being science.
- I've not seen Christians or Jews do this myself, but wouldn't be surprised if there was small bit of this out there for them as well. Usually the Christian Creationist approach is to simply try and show that the text is harmonious with the aspects of the scientific account that can be easily verified; it's only in Islam that I've seen that specific argument being made as a positive sign for the strength of the divinity of the text. But this is just my own anecdotal experience. --Mr.98 (talk) 23:48, 15 February 2011 (UTC)
- Although we don't have a whole article called scientific miracle, there is at least an article section with that title, so I just now created a redirect for it. Red Act (talk) 00:20, 16 February 2011 (UTC)
- I agree with you, and because I'm Muslim, I think I know why such concepts are spreading increasingly in Muslims community. Islam is considered as the final religion and Muslims thus try to make it as consistent as possible with the previous religions but also make it more distinguished by any means. I'm not a judge to assess it but at least I believe that connecting between science and religion is one of the worst tricks used to confuse students in particular. This subject is being studied in some schools and universities in Islamic countries and it was not until I joined University when I started to have a rethink (You can say 99.9% students are brainwashed). I don't like to be emotional but I feel we are somehow responsible if we don't classify this kind of articles. I don't think it is a good idea to make a redirect, because I'm sure other religion have some issues as well but less and hence I'd prefer to generalize the article firstly.--Email4mobile (talk) 00:28, 16 February 2011 (UTC)
- Ok then. I think Scientific foreknowledge in sacred texts article would fit better as a redirect.
- I redirected to the Islam-specific article because the phrase "scientific miracles" appears to at least quite predominantly be a phrase used by Muslims. Some adherents of some other religions have similar beliefs (but about different religious texts, of course), but they appear to instead prefer to use phrases like "biblical inspiration" or "Vedic science". I did also create a "See also" link at the Scientific miracles section to the Scientific foreknowledge in sacred texts article, in case readers want to examine beliefs like that in a broader context than just within Islam.
- The Scientific foreknowledge in sacred texts article, by the way, does already use the word "pseudoscience" multiple times. Red Act (talk) 02:04, 16 February 2011 (UTC)
- It's a very interesting topic, thanks for asking it E4M and for your perspective on it.
- A friend of mine is a Professor at the Center for Islamic Peace (at American University in DC, though she grew up mostly in Egypt and still spends a lot of time in that region) who has absolutely no scientific background whatsoever and yet insists that Islamic scholars have something to say to scientists about Science! It's been almost very annoying at times, to tell you the truth. But I've wondered how widespread this sentiment might be among Muslims more generally, so found your comments above interesting in that regard.
- I do know also that many varieties of fundamentalist Christians put great store in interpreting various translations of various passages in the Christian Bible as prophecies or "miracles" so scientifically sound that even actual scientists would have to admit the absolute Truth of the Word of God if only they weren't such fervent God-haters and -deniers to bother to take the time to scientifically evaluate it (by eg. accepting Jesus as their Lord and Savior and measuring the result).
- Personally btw I think Buddhism is the spiritual practice or "belief system" most "compatible" with Science – Buddhism and science.
- Good luck with expanding an article on this topic though, I'll enjoy having a look at it as it develops. :) WikiDao ☯ 02:25, 16 February 2011 (UTC)
- On Youtube, Thunderf00t just recently did a video on this exact topic in Christianity: [7]. If you don't feel like watching through it or don't have time, here are the two links he gives in the video: one and two, and I also came across these: three and four. This appears to be a relatively minor view in Christianity, but it is not unheard of. TomorrowTime (talk) 08:35, 16 February 2011 (UTC)
- Let's say it is not a problem if the Dr. were not specialized in a scientific field, but the serious problem comes into reality when you hear about a scientific degree Dr. or student preparing researches regarding this subject. See here for example, (Sorry, I Googled the translation from Arabic is I couldn't find English similar one). There you will find a Physicist, or Engineer trying to prepare his PhD in refuting Relativity.If you start with the first lines, you expect this researcher using scientific methods; however, when you reach somewhere in the middle of the paragraph and will be surprised that researcher was trying to find logical answers for the story/miracle of the Night Journey.--Email4mobile (talk) 10:17, 16 February 2011 (UTC)
- Well, that's pretty much the same problem people have with the so-called "Creation science" - namely that it's not even real science but merely a lot of hot air trying to peddle itself as science to people who can't tell the difference. The creation science proponents will, on the other hand, go to great lengths to try and prove that their "science" is legitimate and that there is some vast conspiracy responsible for keeping them out of universities and research labs. You can watch Expelled: No Intelligence Allowed to get a feel for this. TomorrowTime (talk) 15:43, 16 February 2011 (UTC)
- Let's say it is not a problem if the Dr. were not specialized in a scientific field, but the serious problem comes into reality when you hear about a scientific degree Dr. or student preparing researches regarding this subject. See here for example, (Sorry, I Googled the translation from Arabic is I couldn't find English similar one). There you will find a Physicist, or Engineer trying to prepare his PhD in refuting Relativity.If you start with the first lines, you expect this researcher using scientific methods; however, when you reach somewhere in the middle of the paragraph and will be surprised that researcher was trying to find logical answers for the story/miracle of the Night Journey.--Email4mobile (talk) 10:17, 16 February 2011 (UTC)
- This Christian theologian and scientist held that a belief in miracles was atheistic. DuncanHill (talk) 15:51, 16 February 2011 (UTC)
- I really don't understand Baden Powell's point (granted, I should probably read his book rather than his Wikipedia article). I could understand if he'd said that a belief that God performs miracles was incompatible with Christianity. But how can anyone claim that a belief that God performs miracles is atheistic? An atheist can't claim that God performs miracles and remain an atheist. Marnanel (talk) 17:20, 16 February 2011 (UTC)
- I don't know if this is Powell's point, but there's a certain sort of Christianity that treats prayer as a sort of black magic that is supposed to give you what you want, if you wish for it hard enough. The televangelist who, in between ads selling eagle statues to use in prayerful contemplation, tells you about how he can feel someone in West Virginia is praying for little Johnny, and now little Johnny is going to get better. Surely such practices are a mistaken, pagan sort of superstition. (Surely an omniscient God knows of Johnny's pain, and an omnipotent and loving God has a plan to make it so that it never happened, to "wipe away every tear", as they say) Such practices lead inevitably to the belief, antithetical to certain Christian teachings or even the Jewish story of Job, that those who actually do get what they want, who are wealthy and healthy and happy, get these things because they are beloved of God. Now when a person investigates a real miracle, something like the reform against racism in America, a deliverance no less dramatic than the story of Exodus, I think it soon becomes apparent that a miracle is not the achievement of a mundane end by impossible means, but the achievement of an impossible end by mundane means - supplemented only by the intangible, unprovable, but entirely real assistance of God. Wnt (talk) 07:08, 19 February 2011 (UTC)
- I have noticed that, too -- that in certain Christian circles (generally the more "fundamentalist" and/or Evangelical "Mega-Church" adherents, but certainly also in the ritualism of many Roman Catholic practices) what they are essentially practicing is various forms of "magick" plain and simple (though not really too often the "black" variety of that afaik...). I used to mention the similarities between those practices to some of the Christians I know who are like that, which tended to make them very upset, so I've stopped doing that but thought it was nevertheless worth mentioning here. WikiDao ☯ 13:53, 19 February 2011 (UTC)
- I don't know if this is Powell's point, but there's a certain sort of Christianity that treats prayer as a sort of black magic that is supposed to give you what you want, if you wish for it hard enough. The televangelist who, in between ads selling eagle statues to use in prayerful contemplation, tells you about how he can feel someone in West Virginia is praying for little Johnny, and now little Johnny is going to get better. Surely such practices are a mistaken, pagan sort of superstition. (Surely an omniscient God knows of Johnny's pain, and an omnipotent and loving God has a plan to make it so that it never happened, to "wipe away every tear", as they say) Such practices lead inevitably to the belief, antithetical to certain Christian teachings or even the Jewish story of Job, that those who actually do get what they want, who are wealthy and healthy and happy, get these things because they are beloved of God. Now when a person investigates a real miracle, something like the reform against racism in America, a deliverance no less dramatic than the story of Exodus, I think it soon becomes apparent that a miracle is not the achievement of a mundane end by impossible means, but the achievement of an impossible end by mundane means - supplemented only by the intangible, unprovable, but entirely real assistance of God. Wnt (talk) 07:08, 19 February 2011 (UTC)
February 16
Scsbot & SineBot
Can I use these bots in the Arabic Wikipedia questions? If Yes, then what shall I do or can it work by itself there? Because we receive rare questions, we do archiving once a month and this can be done manually but other tasks would be really hectic.--Email4mobile (talk) 01:12, 16 February 2011 (UTC)
Storing DNA for decades
An odd question that ran through my mind this evening:
Let's imagine that somebody wanted to store the DNA of a loved one, child, favorite pet, etc., with the idea that in the future that DNA could be used to create a biological clone of the original organism. (Of course, the clone would not be a duplicate, and would not be identical, except in terms of its nuclear DNA.) What would be the most ideal way to do this from a biological perspective, making conservative guesses about cloning technology advances in the next 20-50 years? E.g. would blood be an ideal fluid, and would it need to be cooled but not frozen, or what? What would prevent or stall the decay of the DNA to the degree that the overall genome would still be constructable? Would the nuclear DNA in the stored cells be actually usable for cloning, or would it require "resequencing" it in some way? I'm not asking for far-out assumptions about cloning, just reasonable scaling up of existing state of the art to the point in which it would be fairly cheap. --Mr.98 (talk) 03:16, 16 February 2011 (UTC)
- Viable cells can be frozen for years, probably decades, in a DMSO/DMEM solution, in liquid nitrogen (<200oC). So sure, you could use those. Even if you were looking tens of thousands of years in the future, beyond the stability of frozen cells or even frozen DNA, you can always reconstruct the genome. A large enough sample can survive arbitrary lengths of time. You can then use deep sequencing to reconstruct the genome. This has been done to reconstruct something like 70% of the Neanderthal genome, even though the only samples were very poorly preserved and tens of thousands of years old. Now, you want 100%, not 70%, but you'll have the benefit of better preservation techniques. Once you have the sequence reconstructed, you'll have to physically reconstruct the chromosomes. That's actually not possible right now in the case of human chromosomes. The record for synthesizing a piece of DNA is a roughly one million base-pair-long chromosome, compared to human chromsomes that can be over 200 million base pairs long. Someguy1221 (talk) 04:11, 16 February 2011 (UTC)
- Unfortunately, no special effort may even be required. Due to policies for prolonged archiving of blood spots on filter paper for dried blood spot testing, obtained from newborns under mandatory laws, de facto DNA database samples exist going back as far as the 1960s These data have apparently been widely available in some cases, even for potential dissemination to insurance companies[8] I am surprised to read that even RNA can be detected on such cards stored at room temperature for 21 years.[9] The uncertain part is what degree of genome assembly can be done in 20-50 years - typical shotgun sequencing allows for construction of whole genome sequences from small fragments, so that part is less of a problem, but currently the reassembly of large numbers of little fragments of sequence into a genome is still a major practical problem. Wnt (talk) 04:15, 16 February 2011 (UTC)
- The last point is a very good one. Nanopore sequencing and similar methods will allow for (theroetically) flawless sequencing of whole chromosomes provided you have at least one intact chromosome in the sample. If the DNA has degraded to a point that no consecutive sequences span a gap...you're screwed unless you can guess what it is. Someguy1221 (talk) 04:21, 16 February 2011 (UTC)
- Blood is not so good because red blood cells don't have nuclei. It would be more reasonable to store hair. But really the most certain way to do it is probably to sequence the DNA and store the data on a CD or DVD. As already noted, we couldn't currently reconstruct the full DNA from that data, but it's pretty conservative to assume that it will be possible in 20-50 years. Looie496 (talk) 04:56, 16 February 2011 (UTC)
- However, blood also contains leukocytes, which do have nuclei. This is the standard source of DNA for most genetic tests. If one is concerned about long term storage of high-quality DNA, the best thing would be to purify it from the blood sample and store it at -80C in a buffer (such as one containing EDTA) that would neutralize any enzymes that might degrade the DNA. On the other hand, if what the OP is after is a source of cells that could be used for somatic cell nuclear transfer or some other type of cloning procedure, then one could generate a cell line from a tissue such as skin, freeze aliquots of those cells and store them in liquid nitrogen. Then, they can be thawed and passaged for whatever future procedure the OP envisions. All of this (except the cloning part, of course) is standard laboratory practice. --- Medical geneticist (talk) 13:48, 16 February 2011 (UTC)
- A science news article today said that a high proportion. nearly 20%, of DNA sequences of animals and vegetables had been found to be contaminated with the DNA of the (human) researchers who handled them. This might interfere with any effort to "reconstruct" some organism from a saved DNA sequence or some blood sample. Your child, brought back via the preserved DNA, might look like the doctor or technician who took or processed the sample. Edison (talk) 03:09, 18 February 2011 (UTC)
- My guess is that by the time 50 years have passed, it will not seem excessive to separate out several individual dried leukocyte nuclei and sequence the entire genome of each one, then compare them. BTW, the most interesting such case may involve a very careful investigation of relics of the True Cross - though, religious matters being such as they are, we would probably get one of the thieves just to serve us right. Wnt (talk) 03:02, 19 February 2011 (UTC)
- A science news article today said that a high proportion. nearly 20%, of DNA sequences of animals and vegetables had been found to be contaminated with the DNA of the (human) researchers who handled them. This might interfere with any effort to "reconstruct" some organism from a saved DNA sequence or some blood sample. Your child, brought back via the preserved DNA, might look like the doctor or technician who took or processed the sample. Edison (talk) 03:09, 18 February 2011 (UTC)
perm press clothes
im wondering why the usa allows clothing to be treated with formaldehyde resin to perm press them. in some places like germany they dont allow this. what gov organization can i contact to complaint about this? — Preceding unsigned comment added by Tomjohnson357 (talk • contribs) 05:42, 16 February 2011 (UTC)
- Depending on the basis for how it might specifically affect you, you might consider a government health agency, an occupational safety agency, or your workers' local union representative. First, you should definitely do some research into the history of that group's position on the matter...nothing worse than saying "hey Senator X, why does the government do Y?" and Sen. X saying "did you read the press release or bill passed a few weeks ago about it?" And also make sure you have some actual science to back up your concern (every public-health issue has tons of false and misleading info out there!). DMacks (talk) 05:55, 16 February 2011 (UTC)
- This article shows that the Government Accountability Office has recently investigated and found it not to be a major problem. It says that Senator Bob Casey instigated the investigation. You could contact him to see if he is continuing the campaign. And this indicates the interest of campaigner Samuel S. Epstein and the Cancer Prevention Coalition. Itsmejudith (talk) 11:43, 16 February 2011 (UTC)
Placebo effect question...
How is it that a patient can take a placebo and it works for them, but then finds out its a placebo and it continues to work for them. I saw a few news articles about this within the past month. I thought once the patient knows its a sugar pill, the effect goes away. 76.169.33.234 (talk) 09:11, 16 February 2011 (UTC)Dave
- Why can't Eastern medicine tap into it as well? Perhaps you meant to simply suggest that "Medicine" should tap into it. -- kainaw™ 14:59, 16 February 2011 (UTC)
- Well, maybe, but I happen to believe that Eastern medicine does tap into it. But as there is no "scientific proof" for my belief, I decided to stick with "Western" medicine. --TammyMoet (talk) 18:10, 16 February 2011 (UTC)
- Whether a scientific fact was discovered in Europe or Africa or Asia may be of great historical interest, (and could potentially be due to strengths or shortcomings of the particular research methodologies used by that culture) - but it has nothing to do with whether the fact is scientifically valid. If there's a particular mechanism of action, and it is describable objectively, then it's moot to label it Eastern or Western medicine, except for purposes of advertising. We have articles on placebo effect and psychosomatic medicine. We also have articles on these exact same topics in Japanese: ja:偽薬, ja:心身医学; and Chinese: zh:安慰劑效應, and in numerous other languages. It should be fair to call those topics "Eastern medicine," if anything deserves that title. However, in common usage in the United States, "Eastern medicine" is often a euphemism for pseudoscience. I think a reputable medical expert should believe in the germ theory of disease and have a solid understanding of modern anatomy (zh:解剖学) and physiology (zh:生理学), as well as the scientific method, irrespective of their country or culture of origin. The placebo effect can be equally well reproduced in the United States and in China. Here's an interesting journal article, Cultural Variations in the Placebo Effect (2000). Nimur (talk) 00:00, 17 February 2011 (UTC)
- Well, maybe, but I happen to believe that Eastern medicine does tap into it. But as there is no "scientific proof" for my belief, I decided to stick with "Western" medicine. --TammyMoet (talk) 18:10, 16 February 2011 (UTC)
- Why can't Eastern medicine tap into it as well? Perhaps you meant to simply suggest that "Medicine" should tap into it. -- kainaw™ 14:59, 16 February 2011 (UTC)
The popular understanding of the placebo effect (the power of the mind to heal the body) is remarkably limited. There is more information at placebo, but the effect can--depending on the patient and treatment--include components of expectation effects, confirmation bias and regression to the mean. The placebo effect is also largely limited to subjective symptoms (e.g., pain and well-being) and is generally not found in objective measures. Conditions for which placebos have the "strongest" effect are typically self-limiting illnesses, which get better over time on their own (the old joke: an untreated cold will go away in a week, but homeopathy will clear it up in only a week!). The other major source of placebo success chronic illnesses that have up-and-down cycles of symptoms (such that a drug taken at the apex of a flareup will appear to have worked if only because the condition would have normalized on its own. — Scientizzle 15:55, 16 February 2011 (UTC)
- Actually, I think that the old joke is that, left untreated, a cold may last up to fourteen days, while advanced medical care can cut that down to just two weeks. Homeopathy really doesn't figure into it. Matt Deres (talk) 16:08, 16 February 2011 (UTC)
- Homeopathy is a prime example of the placebo effect. It figures nicely. — Scientizzle 16:10, 16 February 2011 (UTC)
- I should think it would also be hard to control for other factors when testing for a placebo effect. A person in a hopeful state of mind might alter their behavior from what it would be otherwise, thus introducing additional factors that might favor recovery. Therefore a placebo effect might not strictly speaking be a "power of the mind to heal the body" but rather the effect of introducing factors that favor healing—facilitated by optimism. This is just wp:original research. Bus stop (talk) 16:14, 16 February 2011 (UTC)
- I saw a really interesting article a couple of years ago that not only listed several trials that showed improvements with placebo, but also gave a particular case that seemed to suggest a particular medicine only worked in conjunction with the placebo effect: that is, by itself if did nothing, without it but with expectation, it did nothing, but with both the medicine and a powerful expectation, it worked. Does anyone recall what this was about? 86.164.25.178 (talk) 19:20, 16 February 2011 (UTC)
- Not only that, but more expensive placebos are more effective than otherwise-identical less-expensive counterparts. TenOfAllTrades(talk) 20:43, 16 February 2011 (UTC)
- I think that the new joke is that echinacea is worthless against the flu because it reduces symptoms and severity by about 10 percent, whereas Tamiflu is a vital new drug because it reduces symptoms and severity by about this amount in the 1% of strains which are not yet resistant... Anyway, as for placebos, I think it's important to look at the details. If you take a placebo with a glass of water, this glass of water, twice daily, is a significant therapy (as someone with the gout can tell you with certainty). Wnt (talk) 08:10, 17 February 2011 (UTC)
Proton mass - How standard model can explain ?
The proton mass is estimated around 1 GeV. But we understand that proton is a group of 02 quarks up (mass = 2 eV) and 01 quark down (mass = 5 Mev). It´s suposed that gluon keeps the quarks retained in proton. As gluon is estimated with mass = 0 eV, the addition should be proton mass = 09 MeV. So, How can the total mass from proton be estimated in 1 GeV. From where it cames that mass ? —Preceding unsigned comment added by 177.17.23.86 (talk) 09:56, 16 February 2011 (UTC)
- What you have specified is the bare mass, the effective mass of up quarks in a proton is around 330 MeV/c2 (from up quark#Mass) and the effective mass of down quarks in a proton is around 330 MeV (from down quark#Mass) the difference made up by binding energy. However I would have expected binding energy to be negative, as in nuclei. Graeme Bartlett (talk) 10:47, 16 February 2011 (UTC)
- Yes, most of the proton mass comes from energy -- the energy of the quarks and gluons that make up the proton -- but this energy is positive, not negative. To pull a proton and an antiproton from the void you invested about 2 GeV, now each one has 1 GeV worth of energy "stored in it", hence its mass. This energy cannot be released, as the quarks cannot simply "fly apart"; that is because the strong interaction force between two quarks does not decay with distance like most other forces do. Hope this helps. --Dr Dima (talk) 19:37, 16 February 2011 (UTC)
The Year of the Higgs? A Live Webcast From NSF
Has any of our Science Reference desk volunteers participated [10]?--Email4mobile (talk) 12:19, 16 February 2011 (UTC)
- I have one peer who worked with the ATLAS group and one who is likely finishing her PhD at CERN soon. That said, I really think this all gets a bit over-hyped, not just because I do another more-relevant type of physics, but because I don't think the theory has been developed as extensively as it should be to justify building this gigantic thing in the first place. That said, it's way better than dropping bombs, and I don't believe for a second that one can just not spend the money on this and expect the same amount of funding to be distributed to, oh, say oceanography or geophysics or even, if we want to stay in space, EJSM or IXO. /rant. SamuelRiv (talk) 04:44, 18 February 2011 (UTC)
- The idea that the LHC was built to look for the Higgs boson is basically fiction. The point of the LHC is to explore a new energy regime where there are strong theoretical arguments that some new physics appears. The Higgs boson is considered by far the most likely bet for something the LHC will find, and that may be why it has been hyped so much to the media—the idea is that when the LHC finds the Higgs, that will be seen as "success" by the general public, who are ultimately the ones who decide whether to fund future research in particle physics. It's a con game, in other words, and a somewhat dangerous one since the Higgs might not be found. But most physicists would probably prefer that the LHC exclude the Higgs, because that would be startling and interesting, and would suggest directions for future research instead of reinforcing what we think we already know. More generally, they are desperate for anything that will narrow down the possible candidates for a successor to the Standard Model. The worst possible outcome of the LHC experiment is the outcome that the general public seems to associate with success—that it finds the Higgs and absolutely nothing else.
- As for more-relevant research... what is the point of it? You can build flying cars so that people can commute more quickly to work to do... what? You can cure malaria and AIDS so that people can live longer, healthier lives, giving them time to do... what? Sooner or later people have got to do something other than (do things that make it easier for other people to)n eat, sleep and reproduce, where n ∊ { 0, 1, 2, … }. In practice, the other something is usually watching TV (the large cheap flat-panel TV that you invented), but it might as well be particle physics, especially since there's only one LHC in the world and it cost a lot less than all those TVs. Honestly, I think applied research is great. I'm glad you want to make me happier, but I'm afraid that particle physics makes me happy. -- BenRG (talk) 07:49, 19 February 2011 (UTC)
Is milk disadvantageous?
A balanced intake of all the bone minerals, along with adequate vitamin A, C, and D, is what is truly needed. A balanced intake of minerals cannot occur when the diet emphasizes dairy. Dairy's high calcium causes relative deficiencies in magnesium and other bone-building minerals, and its high phosphorus and animal protein reduces calcium availability. http://www.naturalchild.org/guest/linda_folden_palmer.html Is this true? —Preceding unsigned comment added by 1.23.14.249 (talk) 15:41, 16 February 2011 (UTC)
- That is from a chiropractic doctor who has made a career out of making claims that cow's milk is harmful. Her claims have been refuted repeatedly, but it isn't newsworthy to claim that over 400 years of drinking cow's milk hasn't produced extremely harmful effects. It is only newsworthy to claim that what everyone has done for the last 400 years will kill your baby. -- kainaw™ 15:48, 16 February 2011 (UTC)
- It can be tricky to get a correctly balanced response. On the one hand, saying that milk is "bad" when people have been drinking it for thousands of years seems unwarranted. On the other hand, having it as one of the "four food groups" (though that term is now deprecated and is a redirect) when three quarters or so of the population is lactose intolerant seems more the work of dairy lobbyist groups than actual science. Matt Deres (talk) 16:03, 16 February 2011 (UTC)
- For those without an actual intolerance or milk allergy, dairy has a good record of being a nutritious component of a balanced, healthful diet. For those living in wealthy industrialized nations, the rate of actual vitamin & mineral deficiencies is quite low. The author's simultaneous assertions that milk contains too much calcium but low calcium availability is fatuously absurd. It wouldn't surprise me if the book she's peddling in that article isn't also full of other contradictory and biologically ignorant claims...There's quite a lot of nutritional nonsense out there, and, frankly naturalchild.org looks like it suffers from a severe endemic case of the naturalistic fallacy. — Scientizzle 16:08, 16 February 2011 (UTC)
- I don't think absurd is the right word. For example, I've read claims (from actual scientist-types, not anti-dairy advocates) that the presence of animal fat in milk inhibits the uptake of the calcium (and/or vitamin D). If true (and I'll try to find a cite), that would be a rather large mark against milk consumption, as it's often marketed for its ability to provide those nutrients. I drink milk all the time, but the fact that three quarters of the world is built in such a way that they couldn't drink it even if they wanted to, frankly leaves me suspicious of how great it is. Matt Deres (talk) 19:32, 16 February 2011 (UTC)
- I am not sure of your reasoning Matt. If 75% of the world was allergic to potatoes, would that make potatoes any less of a good food? Googlemeister (talk) 21:59, 16 February 2011 (UTC)
- Well, to those who are in the 75% at least :). I guess my beef (ahem) is with health organizations like those in the US and Canada essentially saying that milk is a required part of a healthy diet - as if the 75% or so of the world that's lactose intolerant is consuming an unhealthy diet. To rephrase your question a bit: if 75% of the world was unable to eat potatoes, how would you feel about the validity of a diet scheme that said they were a necessary part of a healthy diet? That's what I mean about the situation being tricky. Consider dog meat. If we're generous, we'll say that about 25% of the world would consider dog meat perfectly acceptable (it's probably a bit less than that, but it's in the ballpark). If China or Korea released a food pyramid that said that eating dog or dog substitute was a necessary part of a balanced diet, you'd probably look at it a little cross-eyed, but you'd at least admit that red meat is pretty much red meat and dog probably has quite a bit of nutrition in it. But what would your opinion be if most people in the non-dog-meat eating world got violently ill when they tried dog? I think an unbiased observer would say that it obviously couldn't be as necessary as the Koreans or Chinese thought. That's the situation cow milk is in: billions of people never touch the stuff (after reaching childhood), yet there are folks who claim that not drinking milk all your life leads to an unhealthy diet. That doesn't add up for me. Matt Deres (talk) 04:35, 17 February 2011 (UTC) (now stepping off of soapbox)
- Ah, much clarified. Googlemeister (talk) 15:21, 17 February 2011 (UTC)
Homo Sapiens was doing ok. without dairy products since 200,000 years ago right until about 8,000 years ago... Count Iblis (talk) 17:10, 16 February 2011 (UTC)
- Unfortunately, public debate on milk seems to be tainted by industry groups on the one side and animal rights believers on the other. It is already difficult to evaluate whether a food with longstanding use is healthier or less healthy than others, because people vary according to genetics and in what other foods they eat. It is clear that neither cow's milk nor anything else is a satisfactory substitute for human milk for babies, but we vaguely assume adults with a varied diet will hit on a better balanced mix of nutrition by statistical accident or by instinct. Yet obesity and other metabolic variations show that this mechanism isn't working very well, - maybe due to inactive lifestyles, viral infection, epigenetics ... who knows? If that is the case, then is the problem in the milk or in the mechanism by which human hungers are regulated? Wnt (talk) 18:56, 16 February 2011 (UTC)
- There's been several research done about milk. I recall one about a decade ago saying that milk actually removes calcium from your bones, and isn't unthinkable, since osteoporosis is apparently more prominent in societies where milk consumption is high. Vitamin D is also added to aid the absorption, which in combination with adult mammals losing the enzyme to process lactose, point toward the idea that unadulterated milk wasn't really meant for adult consumption. And the research pointing to poor absorption of calcium from supplements made from seashells could suggest that a high calcium content in milk doesn't mean most of it is absorbed by the body any ways. I'll try to find the articles after work if I'm able (currently on lunch break). --Wirbelwindヴィルヴェルヴィント (talk) 20:40, 16 February 2011 (UTC)
- I'd say human breast milk is healthiest for babies, followed by substitutes, like Enfamil, then cow's milk, then real juice, with fake juice and soda-pop being the worst, nutritionally (and soda-pop with artificial sweeteners being the worst of the worst). And, of course, when they don't need food but are just thirsty, water is best. In an older child or adult, it's probably similar (although finding a woman willing to breast feed you might be difficult :-) ), provided you aren't lactose intolerant. Adults also need to worry more about excess calories and fat intake, so that might move all forms of milk down the list, to be replaced by black coffee or tea, at the top, and, perhaps next, alcoholic drinks, in moderation. StuRat (talk) 18:54, 17 February 2011 (UTC)
- I suppose most nutritionists would broadly agree to that ranking, but you really can't make such a thing in a vacuum. For example, even real juice is heavily sweetened with what is euphemistically referred to "grape juice from concentrate" or similar (I can't believe we don't have an article on that; I must not be searching correctly - grape juice is processed to remove the nutrients, the flavour, and much of the water until all that's left is sugary syrup. This can then be labelled as "concentrated grape juice" on the ingredients list so it will look healthier to the public than "sugar" despite being exactly the same thing). Like soft-drinks, the nutritional difference between them and their lower-sugar counterparts depends heavily on circumstances. For most people those empty calories are just an excess, but someone with an otherwise low calorie diet might have need for that sugar water. Matt Deres (talk) 21:29, 17 February 2011 (UTC)
- The trick there is that it is 100% real juice with no added sugar and can say so on the ingredients list. At least "fake" juice will get its sweetness from something like aspartame, allowing you to consume whatever nutrients might be present without requiring you to down a whole lot of empty calories. Even something like Kool-Aid might be better since it will at least make you aware of the sugar content you're doling out. A lot of the literature I read when reading up about childhood nutrition suggested always giving the child water (we're talking kids, not babies, obviously), but providing plenty of whole fruit. Real fruit will contain lots of nutrients and fibre not found in juice (however legitimately made it is) and also won't be adulterated: win-win. Matt Deres (talk) 02:04, 18 February 2011 (UTC)
- I don't think, at least in the US, such a product could be sold as "100% juice", although it could possibly say "contains 100% juice". The grams of sugar are also listed directly on the product bottle, so it's not difficult to determine how much is present. I tend to agree with you about eating whole fruit versus drinking juice, even for adults, but there are times when juice is far more convenient, such as when driving. I don't agree, however, on artificial sweeteners being healthier than sugars. Sugars, in moderation, can be part of a healthy diet, as our body is designed to process them, while artificial sweeteners can break down into toxic components like formaldehyde. StuRat (talk) 19:57, 18 February 2011 (UTC) StuRat (talk) 19:51, 18 February 2011 (UTC)
Shinkansen
If a shinkansen was heading at 500 miles an hour (which it can't) and another shinkansen was heading in the opposite direction at 500 miles an hour, the relative passing speed would be 1000 miles an hour, would there be a sonic boom? --Perseus8235 18:19, 16 February 2011 (UTC)
- It depends how closely they pass. Each train will cause a (subsonic) shock wave, though at 500 mph, this is near the transsonic regime. If the air masses "impact" each other at a relative speed of 1000 mph, a sonic shock will occur; but this would be a really difficult fluid dynamics problem. Transsonic shockwaves are complicated - they behave turbulently, and you're asking about a complicated interaction between two of them. Here are some thorough mathematical treatments: Shock-wave laminar boundary-layer interaction in supercritical transonic flow, (found with a Google Scholar search). Nimur (talk) 18:45, 16 February 2011 (UTC)
- If you want a sonic boom, they could meet in a common tunnel, but they would also most likely damage each other. Googlemeister (talk) 19:18, 16 February 2011 (UTC)
- If the two shinkansen trains approached each other at the speed of sound, I'd expect each to hear a sonic boom from the other, but no sonic boom to be audible to a stationary observer. Since trains aren't all that loud, relative to airplanes, I'd expect the volume of the sonic boom to be less. StuRat (talk) 18:38, 17 February 2011 (UTC)
- Maybe I don't understand how sonic booms work (this is highly probable), but I don't see why the two trains would hear sonic booms from each other, assuming that each train is traveling slightly less than the speed of sound. If a jet flying above the speed of sound flies past a mountain, does the jet hear a sonic boom from the mountain? Maybe the answer is no because the mountain doesn't make noise. In that case my question becomes, if we rig up a big loud speaker system on top of the mountain, does the jet hear a sonic boom then? I would think the answer would be no, since the sound waves from the mountain aren't "piling up" in the atmosphere, so there is no shock wave that the jet passes through. The jet causes a sonic boom to a stationary observer on the mountaintop because it is moving through the atmosphere faster than the speed of sound, not because its relative velocity to the mountain is greater than the speed of sound, right? If my interpretation of the jet/mountain scenario is more or less correct, then I would think that two trains, each moving through the atmosphere slightly less than the speed of sound, would not cause any sonic booms, either to each other or to a stationary observer. —Bkell (talk) 00:12, 18 February 2011 (UTC)
- I would think that the jet moving towards a loud mountain (or any loud stationary object) at the speed of sound would, indeed, hear a sonic boom. The reason is that all the sound coming from the mountain would hit the jet at the same time. Of course, it would need to be a heck of a loudspeaker to replicate the volume of a jet, and anyone playing rap music in his car would be proud to blow out his eardrums (and those of all his neighbors) with that. Also note, though, that a jet has quite a bit of sound protection, so the sonic boom may not be audible inside the jet, over the sound of it's own engines, unless the mountain-top loudspeaker was far louder than the jet. StuRat (talk) 00:24, 18 February 2011 (UTC)
- "All the sound coming from the mountain would hit the jet at the same time"—no, it wouldn't. The sound would be quite Doppler shifted from the perspective of the jet, so the jet will hear the sound at a higher frequency as the jet approaches the mountain, but the peaks of the sound wave will still be separated in the atmosphere by a distance equal to the wavelength of the sound, so the jet will encounter them one after the other, more rapidly than a stationary observer, but not all at once. Imagine a jet and a mountain initially 1236 km apart (the speed of sound in air is about 1236 km/h). Simultaneously, at noon, the jet begins flying toward the mountain at Mach 1, and a very loud speaker starts blasting a sound wave at the top of the mountain. The jet will meet the first sound waves when it is halfway to the mountain, at half past noon; by the time the jet reaches the mountain an hour after noon, it will have heard all of the sound emitted by the speaker in that hour. So the jet hears the sound at a frequency twice that emitted by the speaker (i.e., the jet hears the sound shifted up an octave). At no point in its flight does the jet suddenly hit a "wall" of all the sound energy emitted by the speaker. (On the other hand, after the jet passes the mountain, it hears no more sound, because the sound waves can't catch up to the jet.) —Bkell (talk) 02:17, 18 February 2011 (UTC)
- You're right, but the volume would also increase, in addition to going up in frequency, since it all hits during half the time. If the plane was going mach 10, then 11x as much sound would hit it every second it approached the mountain, at 11x the frequency, and, after it passed, it would hear the sounds backwards, with more recent sounds first, at 9x the normal frequency and volume. StuRat (talk) 19:44, 18 February 2011 (UTC)
- Note that the opposite sort of thing happens from the perspective of the mountain. Let's imagine the same scenario, but with an observer on the mountaintop rather than a speaker system. The observer cannot hear the jet approach before it arrives, because the sound waves cannot travel through the atmosphere faster than the jet; when the jet arrives, so does all the sound the jet has been producing for an hour, and the observer hears a sonic boom. In contrast to the jet's approach, as the jet flies away, the observer can hear it; the sound traveling toward the mountain from the jet will be Doppler shifted down an octave, but it will reach the observer's ears. The difference between the jet's perspective and the mountain's perspective is that the jet is traveling at the speed of sound relative to the atmosphere, which is the medium the sound is traveling in, while the mountain is stationary relative to the atmosphere. —Bkell (talk) 02:28, 18 February 2011 (UTC)
- Guys, this isn't that strange or unintuitive. A shockwave can interact with a stationary object. At normal speeds, we call that an "echo." At supersonic speeds, things are a little different, because supersonic flow means that air isn't behaving the way it "wants" to (it's outside the regime where simple models of pressure waves and laminar flow can apply). Now, the thing you need to keep in mind is "faster than the speed of sound" is a bit of a laymans-description. A better name would be "faster than the equilibrium speed of sound waves, when behaving as normal, linear, propagating waves." Sound barriers are not hard-limits like the speed of light - air particles can and do move faster than the speed of sound, it's just that when they do, their behaviors and interactions are more complicated. What happens when an object is moving supersonically, or even transsonically, is that the object (let's say, the edge of the metal jet) is slamming in to new air molecules all the time. Normally, when traveling at subsonic speeds, the metal of the jet "nudges" air out of the way, and the air molecules can "nudge" fast enough to push other air molecules away - in other words, "a sound wave." Supersonic velocity means that the air is getting "nudged" faster than it can sustainably communicate the kinetic energy to other air - in other words, the metal of the jet is constantly creating a new disturbance with new air molecules.
- Now, when a jet does that at 50,000 feet, far away from anything else, a steady-state equilibrium can be established - a boundary layer of perturbed air, which is called the sonic shock front, takes on a particular shape based on complicated effects of supersonic flow. This represents the capability of the ensemble of air molecules to slam into the jet and (within some characteristic time and length span), convey away the excess energy, eventually decelerating to a sonic speed.
- Suppose another object is "in the way" though - in other words, the shock front is perturbed by a mountain, or another jet, or another sonic or supersonic shockwave, then the effect is going to be a nonlinear superposition of the pressure at each point - because supersonic shocks are outside the linear regime where nice, simple models of sound-wave still apply. We'd get something kind of like an "echo", but not quite - because an echo is a linear superposition of an incident wave and its reflection. So, we could solve the resulting airflow numerically, or we could build a test model and experiment in a supersonic wind-tunnel. Or we could go for a literature search, and find a book, like this one: Supersonic flow and shock waves, to learn all about what's going to happen when a flow interacts with an external object. Nimur (talk) 16:24, 18 February 2011 (UTC)
Chemotherapy drug
How many days does the chemotherapeutic drugs stay in your blood? Does the body try to eliminate it right away after being administered? Is there any add-on, so it stays longer (and therefore kills more budding cancer cells)? Quest09 (talk) 19:08, 16 February 2011 (UTC)
- -There is no universal answer for this, because not only do people's metabolisms differ, but also each drug used. 65.29.47.55 (talk) 21:12, 16 February 2011 (UTC)
- Drugs will usually list a half life for how long they stay in the body. By about 3 to 6 half lives the drug is considered cleared. There are drugs that can interfere with enzymes needed to neutralize certain drugs, but different drugs are neutralized by different enzymes, so there is not one pill that stops all. Some drugs are just excreted unchanged, and don't use enzymes. But taking one of those enzyme blockers can be extremely dangerous! Here's a sampling of some articles that talk about this subject: Grapefruit juice#Drug interactions List of drugs affected by grapefruit Cytochrome P450 (AKA CYP450) CYP3A4 CYP2D6. And here is an example of someone blocking those enzymes to abuse drugs. Ariel. (talk) 23:51, 16 February 2011 (UTC)
Electron Capture
What condition is necessary to have a electron capture by a proton ? In this case could we think that one quark up is changed in a quark down by this electron capture ? — Preceding unsigned comment added by Futurengineer (talk • contribs) 19:09, 16 February 2011 (UTC)
- The total energy after has to equal the total energy before, which is usually impossible because the mass of a hydrogen atom (938.8 MeV) is slightly less than the mass of a free neutron (939.6 MeV). You can think of it as an interaction with one of the three quarks, although strictly speaking, in quantum field theory, no interaction is ever that simple. -- BenRG (talk) 23:26, 16 February 2011 (UTC)
- To complement what BenRG said above, it is usually impossible but there are situations when it can happen:
- Inside neutron deficient nuclei.
- In a collapsing star about to form a neutron star
- Dauto (talk)
- To complement what BenRG said above, it is usually impossible but there are situations when it can happen:
- Don't forget the formation of a neutrino, which affects energy, spin and momentum. Graeme Bartlett (talk) 13:51, 17 February 2011 (UTC)
Hydrocarbon chemistry
What is the opposite of cracking? I am not referring to polymerization where the result is a some huge molecule; I mean something like joining two ethanes to make a butane. Or is this polymerization as well? --T H F S W (T · C · E) 19:20, 16 February 2011 (UTC)
- In principle it's just an example of polymerization in which the reaction is controlled to prevent having a third (or subsequent) monomer unit link into the chain. The term "cracking" is commonly a used to identify a processing stage in petroleum refining. See if Oil refinery#Common process units found in a refinery has anything that sounds like what you want with a more specific term? DMacks (talk) 20:47, 16 February 2011 (UTC)
- I've seen "condensing" being (ab)used in this sense, but please don't ask me for a cite right now. The term dimerisation is used for the restricted sense of the example - combining two monomers. Roger (talk) 07:50, 17 February 2011 (UTC)
- See also Fischer–Tropsch process and Synthetic Liquid Fuels Program. Graeme Bartlett (talk) 08:01, 17 February 2011 (UTC)
Transmission question
how does transmission take place in an axial flow fan having variable blade pitch mechanism? if it is by hydraulic force how the stationary part is connected to rotary part. —Preceding unsigned comment added by 117.201.4.216 (talk) 19:42, 16 February 2011 (UTC)
- Your question is unclear. I suggest you look at Axial fans and Gas turbine. Then you should clarify your question. For example, by transmission do you mean transmission of air through a fan; transmission of torque from an electric motor to the blades of a cooling fan; or something else. I doubt there is any axial flow fan that is driven by a hydraulic motor. Dolphin (t) 06:48, 17 February 2011 (UTC)
- I found a document Consider Variable Pitch Fans which explains their design. If I understand your question correctly, the stationary part is connected (in the example cited in the paper) to the rotary part via a Rotery Air Joint; see figure 7, and the description on pgaes 2 & 3. Come back for more if you find the paper indigetable; I had to read it a number of times before I grokked it. --Tagishsimon (talk) 13:29, 17 February 2011 (UTC)
Electron Capture and Quark Interaction
Since in normal condition electrons don´t decay in proton direction, what are the special condition to cause a electron in K level be captured by a proton ? In this case can we suppose that a quark up is changed in quark down by this electron capture ? In terms of quark interaction what should ocurr ? — Preceding unsigned comment added by Futurengineer (talk • contribs) 20:17, 16 February 2011 (UTC)
Top speed of TRA TEMU1000
What is the top speed of the TRA TEMU1000? --Perseus8235 23:18, 16 February 2011 (UTC)
- According to our Hitachi A-train article, these Taiwanese trains are based on the Japanese 885 series, which have a maximum speed of only 130 km/h but are good on curvy track because they are capable of tilting. Looie496 (talk) 23:29, 16 February 2011 (UTC)
- A Google 3D Warehouse submission gives 150 kph maximum, limited to 130 kph, fwiw. --Tagishsimon (talk) 23:35, 16 February 2011 (UTC)
Adults catch between two to four colds a year and children up to 10 a year????
This statement was made here and I can't believe this. I have had two colds in the last ten years and even people I know who get this a lot more frequently than I don't get this 4 times per year, year after year. When I was a child I got colds more frequently, but surely not ten times per year. Count Iblis (talk) 23:30, 16 February 2011 (UTC)
- Our article on the common cold gives the same numbers, with PMID 4014285 as source for the adult figure. It seems a bit high to me too, though -- even though I know a few people who travel a lot who seem to have a cold every time I run into them. Looie496 (talk) 23:34, 16 February 2011 (UTC)
- Note their use of the weasel words "up to". My 9-year-old probably gets a half dozen colds on an average a year - two or three in the fall and early winter, two or three in the late winter and spring, maybe one or two more spread out the remainder. Matt Deres (talk) 04:41, 17 February 2011 (UTC)
- In my experience, life style has a lot to do with it. People who live and work in buildings that are draught proofed, often overlook the need for getting enough ventilation. This leaves them walking around in an environment with a high virus count. Also, they are unaware of how low the relative humidity can get in heated buildings. This dries out one's nasal airways and eyes, so removing the natural barrier against viruses. As soon as the heating comes on with the approach of winter they all get colds. Tropical fish tanks and house plants can do a lot to offset this but a proper automatic humidifier is worth every cent IMHO. So too is an solid state humidity meter. Later on in the year, if they are not in the habit of spending some time out in the open each day, their body's store of vitamin D can get depleted, leaving them vulnerable to influenza. Breathing cold air also appears to ward off colds. Just travailing to work by motor bike or bicycle seems to give added protection against colds. So, if the OP checks his life style with the people who get frequent colds he might find some differences.--Aspro (talk) 14:35, 17 February 2011 (UTC)
- And note that there's a gray area as to when you have, or do not have, "a cold". Probably everyone has a few cold-causing microbes in them all the time, so the question is how many you need to have to be considered to have a cold or "just the sniffles". Confusion with allergies is also common, and could lead to people reporting more colds than they really had. StuRat (talk) 18:26, 17 February 2011 (UTC)
- I second StuRat's observation, but in the opposite direction; people with hay fever may not notice a minor cold because they're accustomed to sniffles. Comet Tuttle (talk) 20:58, 17 February 2011 (UTC)
- If you live in a small community then you may be exposed to very few new varieties of cold germs every year, and hence already have natural immunisation to most of them. If you meet lots of people in a large cosmopolitan city then you would be exposed to many new varieties of viral infection and hence get lots of colds. Many are transmitted via the hands, so washing hands frequently helps. 92.24.182.65 (talk) 22:03, 17 February 2011 (UTC)
- That seems to assume that many strains never make it into the small community at all. I doubt that this is the case, as the small community would need to be almost totally isolated for this to happen. More likely is that each strain does make it there, carried by mail carriers, delivery men, travelers, visitors, etc., although perhaps not as quickly as to large cities. I'm reminded of the horror film Children of the Corn where all the adults, save one, were killed by the kids in a small town, and nobody seemed to notice for 3 years. The people in that town must have had some rather forgiving creditors, among other things: "I see your mortgage payments are now 3 years overdue, this is your final warning !" :-) StuRat (talk) 19:29, 18 February 2011 (UTC)
- New strains of viral infections are being created by nature all the time, and spread around the world. So it is true that the strains in a small community are only a fraction of those available worldwide. Didnt you ever read or see the news about for example swine flue or bird flue? Or in the past Spanish flue? Don't they teach biology in American schools? 92.29.119.194 (talk) 00:15, 19 February 2011 (UTC)
- That seems to assume that many strains never make it into the small community at all. I doubt that this is the case, as the small community would need to be almost totally isolated for this to happen. More likely is that each strain does make it there, carried by mail carriers, delivery men, travelers, visitors, etc., although perhaps not as quickly as to large cities. I'm reminded of the horror film Children of the Corn where all the adults, save one, were killed by the kids in a small town, and nobody seemed to notice for 3 years. The people in that town must have had some rather forgiving creditors, among other things: "I see your mortgage payments are now 3 years overdue, this is your final warning !" :-) StuRat (talk) 19:29, 18 February 2011 (UTC)
- Nobody has yet linked to our article on the common cold. It has citations for school children getting up to a dozen colds a year. Matt Deres (talk) 14:17, 18 February 2011 (UTC)
February 17
Organic chemistry nomenclature
How are organic compounds named if one of the side chains is branched? For example, what would the name be of the chemical that results when a methyl group is attached to the second carbon of the propyl group in 5-propylnonane? --75.15.161.185 (talk) 01:19, 17 February 2011 (UTC)
- It's all part of IUPAC nomenclature of organic chemistry. You treat the main attachment as itself a parent to which are attached more groups: "(A attached to B) attached to C". When identifying the A on B, with the same rules of group-names, precedence, etc as usual are used, with the additional detail that the carbon in B that attaches to C is considered position #1 in the B sidechain. So instead of propyl, you have 2-methylpropyl, and that whole thing is at position 5 on nonane: 5-{2-methylpropyl)nonane. IUPAC nomenclature. You may also see it named as some relationship to "a four-carbon unit" attached, with some name related to Butyl used to indicate that it's not a literally a -CH2-CH2-CH2-CH3 attached. DMacks (talk) 01:37, 17 February 2011 (UTC)
mDNA
Hi all, I have a question about mDNA populations. If a small group (20) of individuals descended from the same woman (let's call her April), were to assimilate with a large population (20000) that were descended from a different woman (May), am I correct in assuming that the record of April's mDNA would most likely quickly end, and all of the descendents of both groups would eventually only show mDNA for May? Sorry if that's confusing. Let me know, and I'll clarify. One note: I am not even remotely a geneticist. Falconusp t c 04:09, 17 February 2011 (UTC)
- It might disappear but it doesn't have to. If all else is equal, the proportion of mDNA coming from each woman will remain on average constant. There will, however, be random fluctuations, and the proportion coming from April is so small that those random fluctuations may at some point bring it down to zero. This is more likely if the population size decreases, less likely if it increases. Looie496 (talk) 06:33, 17 February 2011 (UTC)
- Gambler's ruin is relevant here. Dauto (talk) 06:55, 17 February 2011 (UTC)
- You've probably already read the article Mitochondrial Eve, but if not it touches on this topic and has further links of possible interest, in particular that on Genetic drift. --87.81.230.195 (talk) 12:06, 17 February 2011 (UTC)
- Some comments:
- 1) You said 20 individuals, but only the number of females in the small group would matter, as the men don't pass on their mitochondrial DNA.
- 2) Note that it doesn't matter whether the women in the smaller group (or the larger group, for that matter), continue to mate within the group or with the other group, it's only the number of female offspring each have that matters for this calculation.
- 3) The chances of the small group of females not having any descendants after a given period of time should be the same if they are in the small group versus the large group, unless being in the larger group either helps or inhibits them from reproducing. It could help if the larger group provides advantages in protection from nature that keeps them alive longer, or it could hurt if the larger group either kills them or prevents them from breeding, as a form of eugenics. StuRat (talk) 17:48, 17 February 2011 (UTC)
Spinning knob, BMW iDrive question
Can spinning the knob on a BMW iDrive (http://www.bmw.com/com/en/newvehicles/3series/sedan/2005/allfacts/_shared/img/ergonomics_idrive.jpg) too fast cause any damage to it? I don't mean spinning it at a ridiculously fast rate, but spinning it very quickly to lets say go through 50 songs at a somewhat fast rate rather than doing it slowly or at a normal rate and doing it for a long period of time (for several months lets say).
- Are you planning to connect it to the drive shaft or something? Turing the knob continuously for several months is very likely to damage it. It probably was only designed to be turned a few dozen times per day and the "bearings" will likely fail, the paint will wear off and particles will get into the sensors. Graeme Bartlett (talk) 13:11, 17 February 2011 (UTC)
- ... but, apart from physical wear, it is extremely unlikely that you will damage the electronics by sending fast signals to the processor. Dbfirs 13:22, 17 February 2011 (UTC)
- It's unlikely to damage the processor. It might crash the software or cause other unexpected effects, but it should be possible to reset the software. Since the iDrive can control electromechanical systems such as CD, it may cause damage to those systems if you repeatedly change the settings (through wear and tear, possibly heat damage, or other methods). It may also void your warranty. --Colapeninsula (talk) 16:13, 17 February 2011 (UTC)
- ... but, apart from physical wear, it is extremely unlikely that you will damage the electronics by sending fast signals to the processor. Dbfirs 13:22, 17 February 2011 (UTC)
I was just wondering about physical wear. My friend spins it very fast and I got upset with her because I'm pretty sure it will damage the bearing overtime. We had an argument over it and she was upset because she thinks it won't damage it and I think it will.
- As long as she is using a fairly gentle touch, I would value friendship more highly than the very slight possibility of damage. This control is new, so there is probably not much evidence of durability in use, but I expect that BMW have thoroughly tested the control on their test benches and designed it to last at least the lifetime of the car, even with heavy use. You don't say whether you are skipping through songs on a CD or on a flash drive, but I expect that the circuitry does not issue a read command until the control indicates a particular track, so there might be lower total wear from skipping quickly through the list. Dbfirs 23:42, 18 February 2011 (UTC)
What is this?

Is it a fish, or amphibian, or what? Its geocoded. Taken in Laem Phak Bia, Ban Laem, Phetchaburi, Thailand at the edge of a river. JJ Harrison (talk) 12:33, 17 February 2011 (UTC)
- That's a mudskipper. It is indeed a fish, amphibian, or what. 81.131.41.98 (talk) 12:40, 17 February 2011 (UTC)
- (EC) It's certainly a fish, more precisely a Mudskipper, but there are very many species of mudskipper and at least two that both live in this general area and have blue spots. The closest visual match I can find after a short google search on Mudskipper Thailand blue spots is Boleophthalmus pectinirostris, but Boleophthalmus boddarti is also a possibility and from the links' text seems more likely to be found in Thailand. --87.81.230.195 (talk) 12:55, 17 February 2011 (UTC)
- Thanks for the information. I'll have a look at the species tomorrow. If it helps it'd be in a Brackish intertidal zone with Mangroves abound. JJ Harrison (talk) 13:17, 17 February 2011 (UTC)
- Oh, from the photos, it looks to be Boleophthalmus boddarti - it has dark brown stripes rather than spots. 58.6.128.181 (talk) 13:19, 17 February 2011 (UTC)
- You should add this photo to the appropriate article; it shows off the colors and anatomy well (at least for the front half of it which is in sharp focus), and it's a pretty specimen. Comet Tuttle (talk) 20:54, 17 February 2011 (UTC)
- Thanks, I intend to. JJ Harrison (talk) 02:40, 18 February 2011 (UTC)
- I concur that Boleophthalmus boddarti is, on reflection, a better visual match of the two species so far suggested, considering that the ones in that link are males displaying, and therefore probably at their showiest, but it would be useful if an ichthyologist could confirm whether your less gaudy specimen is a non-displaying male, a female, or yet a third species. 87.81.230.195 (talk) 08:19, 18 February 2011 (UTC)
- Thanks, I intend to. JJ Harrison (talk) 02:40, 18 February 2011 (UTC)
- You should add this photo to the appropriate article; it shows off the colors and anatomy well (at least for the front half of it which is in sharp focus), and it's a pretty specimen. Comet Tuttle (talk) 20:54, 17 February 2011 (UTC)
Spin Integer
Reading Spin section and basic concept of elementary particles I have some doubts: Since spin of electrons is identified with angular momentum and in consequence causes a magnetic effects, what is concept of spin in photons ? Why we call spin +- n.(1/2) for leptons/quarks and integer spin +- n.1 for bósons ?
- Per the photon article, photons do carry spin angular momentum, and the spin state of a photon corresponds to its circular polarization state. I'm not sure what you're asking with the second question. The unit is h-bar, the reduced Planck constant, and those particles have spin in integer or half-integer multiples of it. Plus and minus are whether the component of spin is parallel or anti-parallel to the direction it's measured in. --Dan Wylie-Sears 2 (talk) 17:39, 17 February 2011 (UTC)
- The OP may be asking a much more in-depth question about the difference between the mathematical formalism of bosons and fermions that is apparent in the integer vs half-integer spins. As I recall, the formalism can be explained in some sense by noting that bosons can be swapped freely with each other, up down in out, having a wavefunction with this symmetry. By contrast, fermions are antisymmetric, flipping their wavefunctions around when swapping places and preventing each other from entering the same quantum state.
- Now here's a really simple illustration of how this makes the spin rule (and this is totally not going to be very accurate in reality): in the symmetric case, wave A adds with wave B to make wave C, but we must make sure we normalize (because wave^2 = 1, since it's a probability), so we get C=(A+B)/sqrt(2), and this seems perfectly natural. If they are fermions, however, the sign flips when the two combine, giving two possibilities for C: (A-B)/sqrt(2) and (B-A)/sqrt(2). But this must again be normalized, so each state only has a contribution of 1/2, so when we extract the angular momentum from the particle linearly and get an integer, a given fermion state gives us a half-integer result. Again, this is totally not accurate, but just an illustration because I'm tired. SamuelRiv (talk) 05:34, 18 February 2011 (UTC)
- I was under the impression that the spins simply add as vectors in three dimensions. The formula for the electron makes sense because it is simply the square root of 1/4 + 1/4 + 1/4 - the result of having a spin of ±1/2 hbar in three different dimensions. What confuses me a bit is that the photon by this logic has a spin of ±1 hbar in two dimensions, and 0 in the third. Looking over circular polarization, elliptical polarization, sinusoidal plane-wave solutions of the electromagnetic wave equation etc., there's always a sense that two independent parameters of the photon's polarization can vary while a third is zero, but I don't really understand it. Looking at various types of photons, which axis has zero angular momentum for each one, and which axes are ±1? Wnt (talk) 02:20, 19 February 2011 (UTC)
- I think you might be trying to extend Fermi spin statistics to spin-1 particles, or perhaps trying to associate each spin state with a given vector, but I'm not sure. A good place to start would be looking at Pauli matrices, where you have σx, σy, σz for the three dimensions. Not that an actual spin state is defined by some combination of all three matrices, and those states are given relative to some reference axis. The associated particles (for spin-1/2) can be in any spin state they want as long as they are all linearly-independent, and as such with the 2x2 matrices there can only ever be two linearly-independent representations, thus spin-up and spin-down, regardless of the actual axes chosen. The 3x3 matrices describe spin-1, which does not make restrict multiple particles from being in the same spin state, but they can be used if you need to define the contributions of spin to each axis of a photon's propagation. Note, however, that the spin is distributed proportionally - there is no single vector of a photon that necessarily gets "more or less" spin. Also note that every spin contribution is 1 - the matrices are unitary. SamuelRiv (talk) 21:12, 19 February 2011 (UTC)
yogurt
Approximately how long would it take for yogurt to go off in a 60 degF environment instead of the 35-40 degF environment of a refrigerator? Googlemeister (talk) 17:13, 17 February 2011 (UTC)
Nonvolatile, Nonflammable oil ?
I want a material which doesn't evaporate and isn't flammable. The application is to mix it with asphalt concrete to keep it from drying out and cracking. I believe shredded tires were used for this in one test case, but then the road caught fire. Of course, I doubt if any oil doesn't evaporate at all or is completely inflammable, so I will settle for an oil or other liquid which can be mixed with asphalt and will evaporate very slowly (over decades) and be only slightly flammable. StuRat (talk) 17:32, 17 February 2011 (UTC)
- Nonflammable lubricants like Krytox might work - but they're pretty expensive if you're planning to mix it in bulk quantities. You should really specify how non-flammable you need: Krytox won't combust even in a pure 100% oxygen environment with a spark present; but that's a little overkill for most purposes. On the other extreme, vegetable oil is cheap and pretty "nonflammable" but can definitely ignite in certain conditions. Nimur (talk) 17:45, 17 February 2011 (UTC)
- Well, I just don't want the road to catch fire. I suppose the worst case scenario is when a gasoline tanker truck has an accident, leaks gasoline all over the road, and it burns off on it's own. That would give us a very high temperature, but just the normal level of oxygen in air and the normal amount of surface area for a road. Also, we must consider that the asphalt concrete won't be 100% this material, but will contain other nonflammable materials, as well. Perhaps a coating of sand on top might both increase friction and decrease flammability, although it would tend to increase tire wear. In this scenario, I'd expect the road under the gasoline to be destroyed, but want to avoid a chain reaction where the whole length of the road burns. So, given these constraints, what material would work ? (If the answer is "none", perhaps "fire breaks" would be needed, in the form of periodic stretches of nonflammable road, like cement.) StuRat (talk) 17:59, 17 February 2011 (UTC)
- What is the exact application?
- Part of degradation of asphalt cement is due to oxidation. Polychlorinated biphenyl would resist oxidization but people like Ralph Nader would get very upset with you. If the surface area is small then you would be better off using an “ Aggregate Bonding Resin” which is usually a polyurethane formula. Epoxy would be best from the non flammability point of view.--Aspro (talk) 17:55, 17 February 2011 (UTC)
- The exact application is a driveway 50 feet long and 8 feet wide. I'm tired of having to do maintenance ever year and rebuild it every few years. The asphalt cement starts out soft, but becomes hard as rock in a few years, so it seems the volatile liquids have all evaporated by then. It is subject to salt exposure and the frost/freeze cycle, as well as direct sunlight and a 2 ton car or truck driving over it about 5 times a day and parking on it 2/3 the time, in roughly the same spot. StuRat (talk) 18:09, 17 February 2011 (UTC)
- Assuming the foundation bed is solid, it might be that your not sealing it with a coat of tar before the asphalt gets laid. There are other stuff on the market sold as "Asphalt Driveway Sealer" which might also mitigate the problem. There are also emulsified tars that one can just sprinkle on from a watering can but I don't know how well they perform. Also, it might not be thick enough, with an aggregate large enough, for the amount of use your subjecting it to. Ask a civil engineer for the right spec. One last thing: Make sure nobody cleans their engine with an 'Engine Degreaser' as they contain oil emulsifiers that ruin asphalt surfaces.--Aspro (talk) 18:30, 17 February 2011 (UTC)
- Yes, the annual maintenance involves applying the sealer in the fall. Quite the hassle, though, as it requires several dry days, is a lot of work to apply, and means I can't use the driveway for several days, forcing me to park on the street. And, even worse, the sealer seems to contain water, and, therefore, as it evaporates, the sealer shrinks and cracks, providing new locations for ice to form in winter. There has to be a better way. StuRat (talk) 19:05, 17 February 2011 (UTC)
- Asphalt Institute has a FAQs page that might hold some answers. Asphalt Pavement Thickness and Mix Design--Aspro (talk) 19:00, 17 February 2011 (UTC)
- At the risk of spamming, products such as this or this might suit your needs. I doubt you're local to that business, but it's apparently part of a much larger chain of businesses that operate under a variety of names in the US and elsewhere. In addition, you could try searching for those product brand names in a more restricted Google search to see if there's a seller near you. (full disclosure: I happen to know the owner) Matt Deres (talk) 19:09, 17 February 2011 (UTC)
- Silicone oil is a nonvolatile, nonflammable oil - but I don't know how it would affect the properties of asphalt. 148.177.1.210 (talk) 19:10, 17 February 2011 (UTC)
- In general, this topic sounds like one of these ideas that can go unbelievably wrong. If you experiment on your driveway with some new chemical, at best it will probably fall apart in some unexpected way, and at worst... well, picture your neighborhood as a Superfund site (after your assets are exhausted). I know that they use silicone oil in McDonald's french fries, but if your neighbor finds it in a test of his drinking water I doubt you're going to convince the court of that without paying a whole lot for lawyers and expert witnesses. Wnt (talk) 19:29, 17 February 2011 (UTC)
- 1) The silicone oil idea sounds like it might work. How cheaply can you buy it in bulk ?
- Epoxy is expensive. Sounds like you need a proper civil engineer to advise you. Such a surface properly applied should last a generation. The tech is tried and tested. Look at the pounding a road undergoes, and they last for a couple of decades before they need resurfacing. --Aspro (talk) 21:45, 17 February 2011 (UTC)
- I largely agree (in fact I was thinking myself it seems strange this is a problem since AFAIK it's not such a big deal for roads etc or even many other driveways which don't need resurfacing every year.) Although it sounds like StuRat has a very unusual driveway. At least I'm not aware of many home driveways where a petrol tanker having an accident and leaking over the road is a concern in any way. For most of us, we'd probably be more worried about the tanker hitting the house anyway in the unlikely event one does have an accident. Nil Einne (talk) 23:12, 17 February 2011 (UTC)
- Just curious but is there a reason you need your driveway to be able to withstand a fuel truck fire? It sounds like overengineering the problem. Googlemeister (talk) 14:27, 18 February 2011 (UTC)
- That's a worst-case scenario. And I said I expected the driveway under the gasoline to be destroyed, but didn't want the whole length to burn, and thus catch the house and neighbor's house on fire. A fuel truck fire may be unlikely, but a car leaking gasoline and starting a fire seems conceivable. I've had a car with a fuel leak before. StuRat (talk) 19:21, 18 February 2011 (UTC)
- Can a road made with recycled tires really sustain an expanding fire? It is true that many of the components of ordinary asphalt are petrochemical, yet it seems to endure many accidents and house fires without trouble. There is very little present to act as a wick. Wnt (talk) 01:29, 19 February 2011 (UTC)
- That's a worst-case scenario. And I said I expected the driveway under the gasoline to be destroyed, but didn't want the whole length to burn, and thus catch the house and neighbor's house on fire. A fuel truck fire may be unlikely, but a car leaking gasoline and starting a fire seems conceivable. I've had a car with a fuel leak before. StuRat (talk) 19:21, 18 February 2011 (UTC)
Chemistry and Lifetime
Hi, I want to study chemistry so it is so important for me to know wether spending much time in labs has a bad effect on health or not.My chemistry teacher says that (on average) chemists live 10 years less than other people because of their contact with chemicals.Is that true?--95.82.49.189 (talk) 18:01, 17 February 2011 (UTC)
- If your chemistry teacher says that chemists live 10 years less than other people because of their contact with chemicals, then he doesn't know what he is talking about. There is no such evidence. At least in industrialized nations, chemists work in environments where their exposure to chemicals in negligible. And even if your job included smelling and tasting chemicals to identify them, I strongly doubt that would have any significant effect on life expectancy unless one routinely worked with extremely toxic/carcinogenic substances. 148.177.1.210 (talk) 19:07, 17 February 2011 (UTC)
- As a trained scientist, you may often be the best-qualified person to evaluate the risks of a particular hazard, be it biological, chemical, radiological, or so on. At the same time, you should always consult Material Safety Data Sheets, EH&S guidelines, and comply with all relevant legal and institutional rules. Working for a university, government lab, or corporate research and development, you will almost always have an on-site Environmental Health and Safety team who are aware of known risks and take steps to mitigate them. Let me also make this abundantly clear: as a scientist, you are never required to deal with hazardous materials; your particular career and research directions may take you where-ever you want, including forays toward the hazardous; but there are plenty of ways to pursue a career in the hard sciences, including chemistry, without ever exposing yourself to anything seriously hazardous. I think it's patently false to suggest that chemists have shorter life-expectancies, on the whole, than other professionals. Unless anyone can show some data to the contrary, I'd say your teacher is incorrect. Nimur (talk) 19:28, 17 February 2011 (UTC)
- Ask for the evidence, as you should in almost everything. It seems rather irresponsible for a teacher to potentially snuff out student interest like that. 66.108.223.179 (talk) 19:55, 17 February 2011 (UTC)
- Answerers, note that the OP is from Iran, where I'm guessing funding for things like fume hoods and dedicated Environmental Health and Safety teams might be tighter than it is in richer countries. At least, their science budget as a whole is quite tight there (see Science and technology in Iran#Budget), which seems like it might make it more tempting there to skimp on safety equipment and procedures. I don't at all know that that's actually the case; my point is just that it's questionable to assume that the job of a chemist is equally safe in all countries. Red Act (talk) 21:01, 17 February 2011 (UTC)
- One thing is that you have to do the statistics with the people already dead, to do the statistics with the students in the 1990s will not give you anything. So lets look at the chemists now 100 years old, they studied in the 1930s and worked in a time when most of the dangers of chemicals where underestimated and some very dangerous chemicals like tetrachloromethane were used as standard solvents. Fume hoods, gloves and other worker safety things were not established in a necessary way. So that these chemists have a shorter live than others should be possible. Now the things a re much better and during my studies I was not allowed to handle for example thallium, cadmium, beryllium and uranium due to safety restrictions.--Stone (talk) 21:48, 17 February 2011 (UTC)
- If anything, a study of chemistry, makes one aware of the true and proportional dangers of chemicals. Reflecting upon what your teacher said, I am reminded of he idiom [12]. A psychologist will also point out, that teachers tend to score high on the neurotic scale. Questioning what you have been told, shows the promising trait of a scientist (or of a preconscious child that should have been strangled at birth). --Aspro (talk) 22:29, 17 February 2011 (UTC)
- This is a Reference Desk, no? Look at Average Age at Death of Scientists in Various Specialties. Male chemists do indeed have an average age at death four years younger than male non-scientists. Male's in radiation sciences do the worst. Notice, however, the small sample sizes and large standard deviations, though it is still possible to draw statistically significant conclusions. The authors point out that those in fields requiring them to be active: archeology and agricultural sciences, for example, tend to do better, probably as a result of getting plenty of exercise. Buddy431 (talk) 23:10, 17 February 2011 (UTC)
- Disclaimer: Buddy431's article is from the year 1969. (Though I salute him for actually supplying a reference.) Comet Tuttle (talk) 23:22, 17 February 2011 (UTC)
- There may be confounding factors in the study (though I concur with Comet Tuttle, and strongly endorse his salute for the use of a proper source). The data are based on deaths reported in Science (the journal) obituaries between 1958 and 1968, which is going to automatically skew the death stats for radiation workers towards a younger age. (The number of radiation researchers would have grown remarkably during the late thirties and onward; few scientists starting their careers during this period would have even reached retirement age by the end of the study, meaning that any death in this group would be 'premature' because the 'natural causes' deaths wouldn't have had time to occur. This effect is even more pronounced among female radiation scientists, where only one woman – 42 years old at time of death – was tabulated.) Interestingly, the average age at death of male radiation workers (Table 1) was tied with administrative workers for the shortest lifetimes—unless filing cabinets are extraordinarily dangerous, it suggests further subtle biases in the dataset. TenOfAllTrades(talk) 02:56, 18 February 2011 (UTC)
- As an electrical worker, I was exposed to several severe electric shocks, high levels of electromagnetic fields, mercury, lead, and solvents such as 1-1-1 trichloroethane, as well as PCB and asbestos. Each of these factors is more likely to shorten lifespan than to lengthen it. I have no doubt that industrial chemists in a lab or factory get exposed to various carcinogins, dangerous chemicals and hazardous conditions at higher levels or frequencies than the general population. Edison (talk) 03:02, 18 February 2011 (UTC)
- This posting contains a list of references that specifically address the question of longevity among chemists. My very brief assessment of the listed studies suggests that the overall mortality rate is lower among chemists than among their peers, but chemists' overall risk of cancers is somewhat higher. An unavoidable challenge in any study of this type is that the data will always be most relevant to the industrial hygiene and health and safety practices of a few decades past. TenOfAllTrades(talk) 03:14, 18 February 2011 (UTC)
- The usual story is that organic chemists in particular once were reduced in lifespan by ten years, but that invention of the fume hood and other precautions put an end to the problem. Though it is widely told, I've never seen a reference to the data - for all I know it's a ruse to sell more fume hoods... Wnt (talk) 14:57, 18 February 2011 (UTC)
- The usual story I heard in school is that it's bimodal...those that don't blow themselves up or poison themselves early in their careers often progress into management or professorships and mostly supervise others from afar rather than continuing to be exposed. But more seriously, modern safety standards definitely have an overall impact (and not just in the MSDS-level paranoia)..."no smoking in lab" (cf. [13]) and the more recent move towards non-heated non-alkali-metal solvent purifications definitely cuts down on ether-still explosions. There might be actual data available for the latter's effect, since lab safety groups are starting to prohibit distillation except in cases where there is no other viable method and companies are charging outrageous prices for Grubbs column setups. DMacks (talk) 17:12, 18 February 2011 (UTC)
Time in a shuttle launch video
Hi all. In the following video: [14] of a compilation of shuttle launches, there is a time counter visible at 0:52 to 1:04. I noticed that it had a weird way of incrementing the seconds. For example, it seems to get to something like 18:31:29 and then goes to 18:32:00. Any idea what this timing scheme is called and why it's used? Thanks. - Akamad (talk) 18:35, 17 February 2011 (UTC)
- And, because the video has been re-processed (transcoded) by Youtube (and possibly by others), some frames may be missing from the raw original 30-frame-per-second stream (roughly speaking, for the purposes of reducing file size).
- Totally unrelated to the video frame counter, NASA's shuttle launch sequences use a non-intuitive timer for countdowns: see "NASA Countdowns" for an explanation of why those clocks don't run exactly "in-order". On most spaceflight missions, there is a Mission Clock, which times "important mission events", (so it stop, restart, and can even run "out of order" in certain cases, particularly during the countdown) and there is also a regular time-clock in UTC that runs perfectly sequentially. Nimur (talk) 19:35, 17 February 2011 (UTC)
- Thanks all. That makes sense. - Akamad (talk) 21:08, 17 February 2011 (UTC)
Gazelle
I remember reading a while back that there was a species of gazelle (or something similar) which slept in 20-30 minute increments. Does someone know the name of the species? 74.15.137.130 (talk) 18:54, 17 February 2011 (UTC)
Thanks! 74.15.137.130 (talk) 16:34, 18 February 2011 (UTC)
Undulatus asperatus
Our article isn't really clear on how they form. Is it vertical wind sheer that causes the strange folds? And what conditions do they develop from and what type of cloud, if any? --T H F S W (T · C · E) 23:46, 17 February 2011 (UTC)
- I believe they are rare enough that the cause is not yet known. Getting them classified as a cloud formation is likely the first step in getting the funds to study them. The last footnote provides this quote: "The Royal Meteorological Society is now gathering detailed weather data for the days and locations where the asperatus clouds have been seen in an attempt to understand exactly what is causing them". If you want speculation, it looks like something is causing them to stay in distinct layers, such as temperature or air-density differences, so that when perturbed by winds, they form ripples instead of mixing evenly. StuRat (talk) 00:01, 18 February 2011 (UTC)
February 18
NMR is a kind of flouresence?
"Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength." vs "Nuclear magnetic resonance (NMR) is an effect whereby magnetic nuclei in a magnetic field absorb and re-emit electromagnetic (EM) energy."
Since radio wave energy is directed towards nuclei in NMR spectroscopy, and radio wave energy is reemitted from the nuclei, one could say that NMR is a kind of flourescence in the radio wave spectrum?
- The term Fluorescence (note spelling) is typically reserved for light absorption and emission arising from electronic energy levels (and indeed, the first sentence of Fluorescence#Photochemistry bears this out) rather than nuclear effects, as in NMR.
L-Glucose
Why haven't food companies replaced regular sugar with L-Glucose? It tastes the same as sugar but it can't be metabolized, so it can't cause weight gain (or cavities, for that matter, since bacteria can't metabolize it either). --75.15.161.185 (talk) 00:09, 18 February 2011 (UTC)
- We have an article on L-Glucose, which says, "L-Glucose was once proposed as a low-calorie sweetener, but was never marketed due to excessive manufacturing costs." —Bkell (talk) 00:17, 18 February 2011 (UTC)
- And from that same article, "also found to be a laxative". Quick google search suggests that it's sweet, but not "same sweetness per amount" so one would have to adjust recipes, and that it does not taste identical, so again would not be a simple swap-in replacement. While those aren't fatal flaws (all artificial sweeteners have similar concerns of sweetness, cooking qualities, solubilities, specific taste characteristics, etc.), the more problems there are, the less likely it is that someone will try to overcome them as well as try to commericalize a more expensive material. DMacks (talk) 01:05, 18 February 2011 (UTC)
Particulate Matter and Heat Convection [?]
I have a bit of a 'thought exercise' concering heat transfer and how it works with such substances as smoke and dust. (It's a problem that is driving me crazy.)
Suppose that there is a solid cube that is open at one end; to wit, 5 of the 6 sides are made of a material that effectively keeps the cold out (eg. gore-tex or polyprolene). And let us suppose that the 6th (open) end of said box is filled up by a thin "screen" of thick smoke or dust making it impossible to see inside. Let us also suppose—for purposes of this exercise—that no matter how hard the wind blows, the "screen" of smoke or dust will not move or blow away; rather, it will remain a thin barrier between the inside and outside of said box.
Now, the temperature inside the box stands at a comfortable 72 degrees (22 Celsius), but the temperature outside the box is a chilly 25 degrees (-4 Celsius). And there is no internal heat source in the box.
Over time, would the temperature inside the box come to match the temperature outside? Or, would the barrier of smoke (or dust) prevent the loss of heat? Also, would the results be any different if the particulates in question were of different substances?
Thank You for reading this! Pine (talk) 00:24, 18 February 2011 (UTC)
- Smoke which is opaque at the visible wavelength isn't necessarily opaque at infrared or other wavelengths, but, I guess, you mean for us to assume that it is. If so, then that would stop all radiation heat transfer, and, since this magic smoke screen somehow resists any attempt to part it, that would also stop convection. However, there would still be conduction, meaning the smoke particles adjacent to the inside of the box would be heated up, then would transfer this heat to particles farther out, by direct contact, until the outside smoke particles were heated up enough to heat the outside air. Conduction is normally quite slow relative to convection and radiation, but you'd still eventually end up with everything the same temperature. StuRat (talk) 00:45, 18 February 2011 (UTC)
- Define "opaque". If the smoke is simply a black body, then it will absorb infrared radiation from the warm surfaces inside the box, and by direct contact with the air, and it will emit infrared radiation also. The radiation it emits will come out in all directions, thereby removing energy from the box. Similarly, smoke that merely scatters light from inside will not prevent energy from escaping. Now if it is both smoke and mirrors (say, a swarm of nanobot corner reflectors tethered in formation) then you have a mirror around a warm spot - essentially a space blanket, effective but not as good as a thermos, since heat is still readily transmitted by contact. Wnt (talk) 18:20, 18 February 2011 (UTC)
Aldehydes and ketones
Why are aldehydes classified as a separate type of compound as ketones, even though they're just 1-ketones? --75.15.161.185 (talk) 00:42, 18 February 2011 (UTC)
- That H allows them to undergo reactions that ketones cannot (example: oxidation to carboxylic acid) and they are generally less stable than ketones (so even the reactions they do that are "same as ketone" are often faster). The H also has distinct properties that have no analog in ketones (spectroscopic signals, chemical reactivity, etc.). At some point, it becomes progressively sillier to give overly specific names based on subtle differences ("ethyl ketone" vs "methyl ketone" perhaps?) but presence vs absence of a certain type of bond or atom is usually important enough to mention. DMacks (talk) 00:59, 18 February 2011 (UTC)
Organism that acts as an air pump
This is a pretty odd question, but I'm hoping someone might have some idea. I'm trying to find some sort of organism (for research), of any size, single or multicellular, that somehow pumps air from one level to another, for example a social algae that removes CO2 from the atmosphere and deposits it underground. It is most important that it transports some sort of gas, though the more/more different types of gas the better. Also if it's a larger organism, structure isn't important, as the research will be built around the organism. Is there anything that fits this bill? Thanks! 64.180.84.184 (talk) 01:28, 18 February 2011 (UTC)
- I don't know if this counts but bees will fan their wings in order to circulate air in the hive. Ants construct their structures with an eye to local winds in order to ventilate the nest. The Mudskipper will dig a hole and keep an air pocket (actually lots of animals will do that). There are animals that collect air bubbles for their nest. Like the Paper Nautilus [16] And here are some more. Ariel. (talk) 01:55, 18 February 2011 (UTC)
- I wonder how much air you could get a diving bell spider to transport if you offer it unlimited food, but sneakily insert a tube into its underwater air supply. But rather than enriching UF6 I'd prefer using an electric eel... ;) Wnt (talk) 03:21, 18 February 2011 (UTC)
Hmm... I didn't even consider organisms that literally transported the air by moving! I don't think this will work though unless the process is passive. 64.180.84.184 (talk) 05:40, 18 February 2011 (UTC)
- Blowfish. Cuddlyable3 (talk) 10:19, 18 February 2011 (UTC)
- Fucus (with pneumatocysts) transport minute amounts of air. I imagine that with just the right environment you could do selection cycles for lumps of fucus that develop buoyancy in the least amount of time - who knows how far it could progress? Wnt (talk) 16:51, 18 February 2011 (UTC)
- Algae often have pyrenoids which actively pump CO2 into them. The article is pretty useless, but this thesis has a lot of details. SmartSE (talk) 17:21, 18 February 2011 (UTC)
- This post on a garden forum describe a slimy algae that rises and sinks in a pond during the day. I would imagine gas is involved in its changing buoyancy, possibly with exchange to the air. EverGreg (talk) 20:21, 18 February 2011 (UTC)
Permeability
what is the value of magnetic permeability(μ) in CGS system of units?—Preceding unsigned comment added by Rk krishna (talk) 05:36, 18 February 2011
- ummm, 1? 213.49.110.218 (talk) 05:45, 18 February 2011 (UTC)
- (EC)The conversion factor from SI units is 4π x 10-7 according to this [17]. Mikenorton (talk) 05:50, 18 February 2011 (UTC)
- This is the conversion from CGS EMU to SI, not from SI. SpinningSpark 01:22, 19 February 2011 (UTC)
- For the record, this is why CGS is and remains awesomely awesome and you blokes who use MKS for your resistors and capacitors ought to be put into forced labor camps until such silly conventions are thoroughly beaten out of you. SamuelRiv (talk) 07:11, 18 February 2011 (UTC)
I changed the section title for easier reference. Cuddlyable3 (talk) 10:14, 18 February 2011 (UTC)
- There are (at least) two versions of the CGS system when it comes to electromagnetic quantities: namely the electrostatic (ESU) and the electromagnetic (EMU) system of units (there are others, but they have the same result as EMU for permeability). In the EMU system, as stated by 213.49.110.218 above, the value of μ0 is 1. In the ESU system, however, it is approximately . See this table. SpinningSpark 15:41, 18 February 2011 (UTC)
fridge
how come the engine dosent burn out on those open fridges in stores that have yogurt ect theres no door
- They make the engine larger so it can handle the increased demand. Then it can cycle on and off like a normal compressor. And BTW, I don't think they would burn out even if you did run them continuously. Starting is probably the action that wears a motor the most, just letting it run doesn't harm it (much). Ariel. (talk) 07:18, 18 February 2011 (UTC)
so can i leave my fridge open for weeks?
- Without burning out the motor (which will run continuously), probably yes. But since your household fridge (presumably) wasn't designed to work with the door open, it won't be able to achieve a normal fridge temperature, will consume a lot more electricity for which you will pay, and will likely ice up quickly and have to be defrosted. Stores have to balance increased power consumption (and larger and more capitally expensive motors) against the convenience of their customers not having to look through, and open and close, doors, which would reduce sales and revenue. Also, an open fridge markedly cools the air in its vicinity, which is acceptable for customers briefly visiting an area of a store, but would likely be uncomfortable in your home. 87.81.230.195 (talk) 08:13, 18 February 2011 (UTC)
- ... and many supermarket fridges have closed glass doors. The open ones are designed to reduce escape of cold air (unlike household fridges). Dbfirs 08:41, 18 February 2011 (UTC)
- Note that leaving open the door of your kitchen refrigerator for weeks is a way of making the kitchen warmer not cooler, and is likely to spoil your yogurt, etc. (short for et cetera) Cuddlyable3 (talk) 10:11, 18 February 2011 (UTC)
- True: I was unconsciously assuming extraction of the warmed air, which is unlikely to be installed on a household fridge. 87.81.230.195 (talk) 15:15, 18 February 2011 (UTC)
- When I worked at a grocery store, back in my teenage years, the coolers with the open front were designed where cold air blew out a vent in the front and was aimed up and back. This seemed to make a kind of barrier that helped to prevent the cold air already in the cooler from escaping easily. Googlemeister (talk) 14:23, 18 February 2011 (UTC)
- Note that leaving open the door of your kitchen refrigerator for weeks is a way of making the kitchen warmer not cooler, and is likely to spoil your yogurt, etc. (short for et cetera) Cuddlyable3 (talk) 10:11, 18 February 2011 (UTC)
- ... and many supermarket fridges have closed glass doors. The open ones are designed to reduce escape of cold air (unlike household fridges). Dbfirs 08:41, 18 February 2011 (UTC)
biochemistry
the synthesis of ATP by photosynthetic system is termed as????
 Please do your own homework.
Please do your own homework.- Welcome to the Wikipedia Reference Desk. Your question appears to be a homework question. I apologize if this is a misinterpretation, but it is our aim here not to do people's homework for them, but to merely aid them in doing it themselves. Letting someone else do your homework does not help you learn nearly as much as doing it yourself. Please attempt to solve the problem or answer the question yourself first. If you need help with a specific part of your homework, feel free to tell us where you are stuck and ask for help. If you need help grasping the concept of a problem, by all means let us know. -- P.S. Did you see our article on photosynthesis? ---- 174.21.250.120 (talk) 16:37, 18 February 2011 (UTC)
Blocking gravity?
Is it impossible to block gravity or didn't we just discovered how to do it? If it's impossible, what does make it impossible? — Preceding unsigned comment added by 83.54.216.128 (talk • contribs)
- One of the reasons that it's impossible is that there is no such thing as negative (gravitational) mass. Technically speaking, you can't "block" the electrostatic force either. However, what you can do is counter the attractive/repulsive force with an equivalent amount of opposite charges. That's why there's no electrostatic attraction between the earth and the moon, despite the electrostatic force being many orders of magnitude stronger than the gravitational force. Protons equal electrons and the net charges are zero. The materials which "block" electrical fields do so by reacting to the electrical field, setting up their own, opposite direction field, which cancels out the original field. This they do by separating the positive and negative charges. There's no such thing as negative mass, so you can't counter gravity with an equivalent amount of it, nor can you set up an opposing field with positive/negative mass separation. -- 174.21.250.120 (talk) 16:08, 18 February 2011 (UTC)
- The current understanding of gravity doesn't allow for it to be blocked. But, science fiction does because it can consider gravity to be transmitted by particles called gravitons. Because they are particles, you can block them. Then, gravity cannot be transmitted. The problem with this is that every object would emit gravitons. So, if you place a huge gravity shield between you and a planet to block gravity, you'd then be attracted to the huge shield. -- kainaw™ 14:07, 18 February 2011 (UTC)
- Though one can imagine science fiction scenarios whereby the gravitons are deflected by means of something massless. And if the deflection device was of considerably lesser mass than the thing it was deflecting, that would still be pretty useful. But I'm not saying that any of this is really science. --Mr.98 (talk) 14:55, 18 February 2011 (UTC)
- You might find antigravity and gravitational shielding a good place to start. Among other things, such devices might allow construction of perpetual motion devices—something that the Universe generally frowns upon. TenOfAllTrades(talk) 14:11, 18 February 2011 (UTC)
- One possible form of dark energy (the one which I believe in) is something in galactic voids which exerts an anti-gravity force and thus both pushes galaxies together and also increases the rate of the expansion of the universe. (A different form of this theory has the dark energy constant everywhere, which still accounts for the accelerating expansion of the universe, but wouldn't force galaxies together as much.) If either form of the theory is correct, then it might, in the distant future, be possible to concentrate the source of this dark energy to create a strong anti-gravity field in a certain location, strong enough to cancel the gravity of a planet or star. StuRat (talk) 18:52, 18 February 2011 (UTC)
- Particles aren't in my field (pun), but gluons are self-interacting strictly-attractive particles and thus, if produced freely, can block each other. The point is merely that there are, in accepted theory, ways to affect a field with certain properties similar to gravity, so not knowing really anything about the gravitational charge-carrier graviton is quite a hinderance to a definitive answer to your question. We are safe in saying, however, that nothing within what we currently know would do such a thing.
- One important thing with speculative/science fiction on anything like antigravity, however, is to make sure it doesn't violate more fundamental laws of nature, particularly conservation of energy. When you start puncturing a bunch of holes and tubes in spacetime, we can talk about local violations of this law, but otherwise it has to be obeyed by the very definition of the universe itself. SamuelRiv (talk) 21:32, 19 February 2011 (UTC)
Atom
Are there any circumstances where the distance from nucleus to electron shell within the same atom changes? — Preceding unsigned comment added by 165.212.189.187 (talk)
1. I dont know yet. OK, Is the distance from the inner/outer (take your pick) electron shell to the nucleus constant under all possible conditions in which a particular atom can exist? or can it be different depending on certain circumstances? — Preceding unsigned comment added by 165.212.189.187 (talk)
- If you place an atom in an electric field the electron will preferentially be found on one side of the nucleus (polarisation). I'm not sure whether that changes the average distance of the electron from the nucleus. --Wrongfilter (talk) 16:16, 18 February 2011 (UTC)
- Also, if the atom is part of a molecule the electron may spread out into a molecular orbital which is larger than a atomic orbital. Dauto (talk) 16:22, 18 February 2011 (UTC)
- First off, the concept of "distances" in atoms is a little undefined. Because of the quantum nature of subatomic particles, electrons are simultaneously infinitesimally close to the nucleus and infinitely far from the nucleus. The probability varies by radius and is vanishingly small at the extremes, so there's a bounded region where most of the probability occurs, but that region doesn't have a sharp boundary. (Where do you place the inclusion cutoff? 50% probability 90%? 95%?) That said, the shape and location of the probability distribution is effectively governed by the mass of the electron, the amount of energy it has, the number of electrons it's sharing its space with (cf. Pauli exclusion principle) and the electromagnetic forces acting on it. In the ground state and the absence of an external electromagnetic field, the electromagnetic forces are determined by the number of protons in the nucleus (which element it is), and the number of electrons. This means when you ionize an atom, you change the electron shells - not just the shell which the electron is added/removed from, but all the electron shells. The amount of energy is also important. If you excite an electron (say by it absorbing light), it changes its probability density. This is usually approximated by saying the electron jumps to a different electron shell, but there's a reorganization of all of the shapes and sizes of all of the electron clouds. If you're talking about atoms in molecules instead of isolated atoms, you throw in a host of other complications, as you're not only adding in extra electrons, you have "external" fields from the other atoms in the molecule. (Complicated to the extent that molecular orbitals (electron shells) are more than just a collection of "atom orbitals" and "bond orbitals".) - All that said, within any particular set of conditions, the electron shells should be of constant size and shape. That is, for example, a ground state, neutrally charged neon atom in the absence of any external electromagnetic field will have electron shells exactly the same as any other ground state, neutrally charged neon atom in the absence of any external electromagnetic field. -- 174.21.250.120 (talk) 16:36, 18 February 2011 (UTC)
- How can an electron be "infinitely far" from anything, let alone the nucleus? Matt Deres (talk) 17:37, 18 February 2011 (UTC)
- The "location" is described as a continuous probability function. Any finite limit you place on the distance would be an arbitrary cutoff on that function. The value of the function assymptotically may approach zero, but it doesn't actually permanently reach zero at a certain definite distance out, so again you can't say you will only consider it to be defined up to that certain distance. Now practically speaking, it might be more useful to say "arbitrarily" (since we are using the fiction of electrons having definite position) instead of "infinitely". DMacks (talk) 17:50, 18 February 2011 (UTC)
- I understand that you can't know the position of an electron, but I thought the continuous probability function was for the location "on" the specific shell configuration, or at least that the outermost point of the outer shell was the limit of distance that it could be from the nucleus. Are you saying that the outermost point that an electron can be from the nucleus is infinity? — Preceding unsigned comment added by 165.212.189.187 (talk)
- Yes, that is the mathematical result when you compute the probablity distribution for an isolated atom, in a Universe which contains just this one atom. This probablity distribution will have to be modified at distances where the electron feels the influence of another atom. Even in the idealised case, the probability drops very quickly, so even if it is finite even at a kilometer from the nucleus, it is very small indeed. --Wrongfilter (talk) 21:50, 18 February 2011 (UTC)
- I understand that you can't know the position of an electron, but I thought the continuous probability function was for the location "on" the specific shell configuration, or at least that the outermost point of the outer shell was the limit of distance that it could be from the nucleus. Are you saying that the outermost point that an electron can be from the nucleus is infinity? — Preceding unsigned comment added by 165.212.189.187 (talk)
- The "location" is described as a continuous probability function. Any finite limit you place on the distance would be an arbitrary cutoff on that function. The value of the function assymptotically may approach zero, but it doesn't actually permanently reach zero at a certain definite distance out, so again you can't say you will only consider it to be defined up to that certain distance. Now practically speaking, it might be more useful to say "arbitrarily" (since we are using the fiction of electrons having definite position) instead of "infinitely". DMacks (talk) 17:50, 18 February 2011 (UTC)
- How can an electron be "infinitely far" from anything, let alone the nucleus? Matt Deres (talk) 17:37, 18 February 2011 (UTC)
You can also "encourage" the electron in a given valence shell to be at a greater distance (higher energy) from the atom without changing the quantum state. This happens in intermolecular interactions all the time - see London dispersion forces. SamuelRiv (talk) 21:38, 19 February 2011 (UTC)
Cost of Driving
I'm trying to figure out the marginal cost of driving a car. It seems the total cost is a function of both mileage and time. Some costs are obviously related to the miles driven, such as gas, and others are obviously independent of mileage, such as the insurance costs. The cost that has me stumped is depreciation. The car loses value both as a function of time (the car will lose value over time whether or not you drive it) and mileage (two cars of identical age but with different mileage are worth different amounts). Does anyone know how to quantify this? anonymous6494 14:53, 18 February 2011 (UTC)
- It's also dependent on how popular the particular model is on the second-hand market, which is to some extent influenced arbitrarily by fashion and therefore fluctuates over time - some models become more valuable with age, assuming no unusual deterioration in condition. In the UK, at least, cheap guidebook-type magazines are widely published and frequently updated listing averages of current values, based on recent sales, and there are on-line equivalents such as this.
- Alternatively, an accountant valuing the depreciation of a company-owned car might just assign an arbitrary percentage-of-the-original-cost loss per year, such that in x years the value on the company's books will decline to zero, but as that linked article (which actually uses a vehicle as an example) details, there are alternative methods. 87.81.230.195 (talk) 15:12, 18 February 2011 (UTC)
- I had looked at Kelley Blue Book's website to try various combinations of age and mileage but the resolution was not very good (a large change in mileage was needed to produce a change in value). The site you linked was similar but my car isn't listed so it may not be available in the UK (it may be there under a different model name). From an accounting perspective US tax law makes it clear how to depreciate a motor vehicle used for business, but depreciation doesn't accurately depict the actual value, only its value for tax purposes. I was hoping to determine how the actual value is affected by driving additional miles. anonymous6494 15:30, 18 February 2011 (UTC)
- Glass's Guide gives the mileage adjustments for the retail and trade values of each model and age of car in the UK. The figure depends on the model and age of the vehicle. I don't have access to a copy to cite any figures, but last time I checked for my old car it was around 5p per mile. It will probable be double that for current models. Dbfirs 17:41, 18 February 2011 (UTC)
- Note that your example of a cost which is independent of mileage isn't quite right. Insurance companies often ask how many miles you drive per year and figure that into the premium price. Also, if you drive more, an accident is more likely, and if this leads to a claim then your rates will go up again. Finally, if you drive more, tickets are more likely, again increasing your premiums. StuRat (talk) 18:39, 18 February 2011 (UTC)
- Yes, good point. If you get quotes for different anticipated mileages, you can split insurance into fixed and variable elements, though I expect that most insurance companies will use a step function rather than continuously variable premium. Dbfirs 23:45, 18 February 2011 (UTC)
- There are some basic rules in behavioral economics that, all variables regarding car make, model, condition, etc being equal, might give you some guidance on how much a car depreciates in people's heads. For example, let's quantify the idea of a new car losing half its value as soon as its driven out of the lot. This might seem an irrational cutoff, since the car's been test-driven plenty, often by people who don't know how to use the clutch properly. However, given the choice between a guy offering a discount of $100 on a $10k car (1%) after driving it for a week after buying it and deciding he didn't like it, versus buying a car "new" with no discount, even if the mileage were the same, most people would buy the "new" car. Why? Because it's "new", and 1% isn't a lot to spend (versus, say, for a "new" $300 TV versus a one-week used $200 TV, most would buy the used one at a 33% discount, even though the proper discount and marginal utility are about the same between a car and a TV). My suggestion is, if doing this informally, come up with a list of survey questions, such as "The car is new on the lot, but the same car with same mileage used for one week is for sale at __% off, which do you buy?", and refine the price as you ask more people - you probably only need to ask 5 or 8 people before getting a pretty good idea of how a car depreciates over each year for 10 years, as long as you don't ask them about multiple years in succession. And of course, your best source on behavior is yourself, as long as you answer yourself honestly. SamuelRiv (talk) 21:49, 19 February 2011 (UTC)
Food spoilage - refreezing meat
One of the mantras of food safety is (so far as I'm aware) to never re-freeze meat products, presumably because the bacterial load increases dramatically during the second thawing cycle. Is there something intrinsic to the freeze-thaw cycle that favours undue bacterial proliferation? If I take any given two pounds of ground beef, freeze and thaw one of them twice over the course of two days, and just leave the other one in the fridge for two days, will there be any appreciable difference in the multiplier of bacteria count? I'm aware of the need for proper cooking, proper handling to avoid cross-contamination, &c, but can't find any biological basis for this particular "rule" of food in our articles. (FD: this pertains to a real-world discussion, but the discussion is with a medical doctor, so at least on this end any suggestion of medadvice would be met with peals of laughter - I'm asking about only the biology here) Franamax (talk) 16:01, 18 February 2011 (UTC)
- I've honestly never heard of this particular "rule." The Food Safety and Inspection Service is the king of meat food safety here in the US and A, and their advice page on freezing doesn't say a thing about it. Even listeria doesn't grow at freezing temperatures since the water activity of ice is... marginal. Foods that are handled a lot (i.e. if you take a little bit off a hunk of ground beef six or seven times) are at greater risk just because every encounter adds a possibility of contamination, but washing your hands and other common sense stuff makes that risk marginal. To be honest, cooking it fully is the big answer to most bacterial issues, though S. aureus toxin is of course going to stick around even if the bug itself is gone. The quality of thawed and re-frozen meat might be... undesirable, of course. SDY (talk) 16:16, 18 February 2011 (UTC)
- The American FSIS say on their website that it's ok.[18] But the Food and Nutrition Service say it's not.[19] The UK's Food Standards Agency says you can refreeze meat once cooked, not raw.[20] In the EU, the statement "Do not refreeze after defrosting" is mandated on all quick-frozen goods under Council Directive 89/108/EEC. 17:04, 18 February 2011 (UTC)
- And The Straight Dope has a quick overview. Nanonic (talk) 17:12, 18 February 2011 (UTC)
- All frozen food in the UK carries this instruction not to refreeze. Its serves as simplified guidance to hoi polloi who may not understand or follow more complex advice. Considering how dangerous food poisoning is, it seems a sensible approach. Much of the fresh unfrozen meat and seafood that you buy in supermarkets was stored in deep freezers, yet it has been thawed and often labelled as being suitable for freezing on the day of purchase. Chefs etc., on the other-hand, are schooled in the health and safety aspects of food storage and should know when its safe to refreeze. Their fridges and freezers must also have externally visible temp gauges and have fans to speed up cooling.--Aspro (talk) 17:19, 18 February 2011 (UTC)
- It's not just the time of the freezing process, it's the total time of the meat spent at temperatures that are compatible with bacterial growth. If the meat spends four hours at susceptible temperature thawing, then two refreezing, then four hours thawing again, that's sort of like leaving it out for ten hours, and you may end with so much pathogen that even if cooking kills 99.9% of it you still have enough for an infectious dose. The "don't refreeze" isn't ridiculous in the context of cumulative time, but "don't leave it sitting at temperatures that grow bacteria" is really what they're talking about. SDY (talk) 17:55, 18 February 2011 (UTC)
- I think the problem may not be with the bacteria themselves, but our perceptions and memories. Consider three paths frozen meat can take:
- A) You buy it frozen, thaw it, leave it in the fridge too long, and you get worried about it going bad soon, so you cook it and eat it. Perhaps you have a light exposure to bacterial toxins.
- B) You buy it frozen, thaw it, leave it in the fridge too long, and you get worried about it going bad soon, so you toss it. No exposure to bacterial toxins.
- C) You buy it frozen, thaw it, leave it in the fridge too long, and you get worried about it going bad soon, so you refreeze it to stop the bacterial growth. It then stays in the freezer for a year, and you forget that it was questionable when you refroze it. You now take it out, thaw it, and leave it in the fridge several more days, and it goes bad before you cook it and eat it. Heavy exposure to bacterial toxins.
- This could be addressed by writing the history of how long the meat has been stored at various temps on a label on the meat, and doing some calculations to see if it's still safe, but most people wouldn't do that (perhaps a butcher dealing with sides of beef might). Another factor is if the meat is defrosted in warm water, which is a bacterium's dream. Doing this twice is far worse than once (if the bacteria grows to 10x the original count once, it would grow to 100x if this is done twice).
- Perhaps in the future each slab of meat will come with a device that can tell you whether it's safe or not, by, say, changing the color on an indicator strip that darkens with time and temperature, paralleling the growth of bacteria. This could also be done with a reusable digital thermometer and clock combo. It would ideally be reset and then kept with the meat from the time of the slaughter until cooked and served. I picture them being returned to the butcher/store for refund at the next visit, and then returned to the slaughterhouse, sterilized, reset and reused. Somewhat less effective would be if the consumer took his own device with him to the store, put it with the meat as he put it in the basket, and reset the device then. StuRat (talk) 18:09, 18 February 2011 (UTC)
- Thanks all for the info and the links. I read them and they seem to repeat the "mantra" without substantiation. However, I may have come up with the answer on my oen. When cells are frozen in uncontrolled situations, they have a tendency to lyse, I believe during tha freezing process but only made evient upon thawing. This is the difference between selecting only those bacteria competent to penetrate cell membranes and putting a come-one-come-all sign out on the street, isn't it? Is there a reliable source to confirm that? Franamax (talk) 18:35, 18 February 2011 (UTC)
- For food safety, bacterial growth is primarily about four things: nutrients, water, time and temperature (there are other potential complications like pH, but they're not a factor with meat). If the lysed cells provide better nutrition for the bacteria (plausible) or more available water to support growth that might help, but the non-refreezing argument appears to be mostly targeted towards time and temperature. SDY (talk) 18:56, 18 February 2011 (UTC)
- It's going to be difficult to find a reliable source that will tell you its okay to refreeze meat. As you've no doubt gathered from the responses above, there is at least a non-zero risk every time product is brought to room temperature (i.e. the danger zone). If "common wisdom" says that you should only freeze meat once, any source advocating multiple freezings would leave themselves legally/morally liable for any misadventure that occurred as a result - and for what? I can't think of a particular person or group that would have anything to gain from people refreezing their goods (other than maybe the manufacturers of freezer bags). And when you factor in how poorly most people understand the vectors of food-borne illness plus the ill-conceived methods many people employ to defrost food (e.g. leave it on the table all day for supper that night), you start to think that giving folks the dumbed-down (but safer) advice isn't such a bad thing. Matt Deres (talk) 19:20, 18 February 2011 (UTC)
- Thanks all for the info and the links. I read them and they seem to repeat the "mantra" without substantiation. However, I may have come up with the answer on my oen. When cells are frozen in uncontrolled situations, they have a tendency to lyse, I believe during tha freezing process but only made evient upon thawing. This is the difference between selecting only those bacteria competent to penetrate cell membranes and putting a come-one-come-all sign out on the street, isn't it? Is there a reliable source to confirm that? Franamax (talk) 18:35, 18 February 2011 (UTC)
- I wasn't aware that avoiding refreezing is a safety matter. I thought the point is that when you freeze and thaw meat multiple times, the texture turns more and more mushy. Looie496 (talk) 20:45, 18 February 2011 (UTC)
- Well, it's a safety matter in the sense that bacterial growth is greatest when food is in the danger zone. When the food is sitting in your freezer, bacterial growth is greatly restricted due to the water being unavailable for use. When the food is sitting in your oven, the internal temperature of the food is (hopefully) being raised to the point where the bacteria are killed off. But room temperature is problematic and that's the temperature many people defrost their meat at. For example, when I was a kid, my mom thought nothing of leaving a package of ground beef on the counter for several hours so she could use it for supper. Likewise with cuts of chicken, pork, and so on. Do that once and you might get away with it, but each time you do it, you roll those dice again. For the record, the best way to defrost a chunk of meat is to immerse it in cold water that is kept in motion, for example by putting it in a bowl filled with water and with the tap set to drizzle cold water in, keeping a current moving. Even big chunks of roast defrost quickly - and without the partially cooked bits you get from using a microwave.
- No question that the texture gets worse - and not just with meats. Stuff like strawberries will literally turn to mush after just one or two re-freezings. Matt Deres (talk) 21:27, 18 February 2011 (UTC)
- It sounds like your mother may have known a lot about how to avoid food poisoning. Before refrigeration or ice boxes came into being, people knew what was good and bad practice. Even today, in places like Africa, one can see raw red meat covered black with flies and on the point of going putrid, but cooked -its OK. Chicken there however, is taken home still flapping and vocally protesting its innocence. Also, I have noticed that in recent years red meat in our own supermarkets is not so well bled as it used to be. This shortens the time it can remain safely uncooked. Well bled beef tastes much better if it is matured for a few weeks before cooking. The Inuit actually eat putrid meat as a delicacy... but I will leave that until another day. --Aspro (talk) 22:07, 18 February 2011 (UTC)
- Something that hasn't been mentioned yet is that the bacterial toxins have more time to build up with repeated thawing and refreezing, and they are not removed by cooking. 92.29.119.194 (talk) 00:19, 19 February 2011 (UTC)
Do other apes have problems giving birth?
Does any other species have so much trouble? 66.108.223.179 (talk) 16:03, 18 February 2011 (UTC)
- Obstetrical Dilemma is relevant but a little vague about when exactly humans' ancestors developed bipedalism. Comet Tuttle (talk) 18:09, 18 February 2011 (UTC)
- It was confirmed a few days ago that Lucy's species walked upright as humans do 3 million years ago. 66.108.223.179 (talk) 05:28, 19 February 2011 (UTC)
Virtual black box
Aircraft black boxes provide valuable crash info, but can't always be recovered. Has anyone considered the option of a virtual black box ? It would work like this:
1) During operation, airplanes would broadcast their current black box info, at a designated frequency, to the nearest tower. This signal would include identification info for the flight and airplane.
2) A computer at the tower would then store this info.
3) We could stop here, and have investigators contact the various towers near the flight path to retrieve the info after a crash. Or, the towers could report the info, in turn, via the Internet, to a central site where the records for each flight are accumulated and available for real-time analysis on all flights. This could be useful, say, to identify a systemic problem like wind sheer or multiple hijackings, while the info could still be used to prevent further problems with other flights.
I envision this system being in addition to the current black boxes. So, has anyone proposed this ? StuRat (talk) 18:27, 18 February 2011 (UTC)
- (ecx2) Here's a proposal that was published in IEEE Spectrum. You'd still need the black-box flight data recorder to deal with transoceanic flights, over-the-Amazon flights, etc. Comet Tuttle (talk) 18:35, 18 February 2011 (UTC)
- Aircraft Communications Addressing and Reporting System can be used to download some aircraft data to ground stations, with the dissapearance of Air France Flight 447 it has provided some data to the investigation as the aircraft and the aircraft data recorders have not been found. MilborneOne (talk) 18:43, 18 February 2011 (UTC)
There is the problem that each tower can only track airplanes within a limited range, due to their radar being blocked by the curvature of the Earth. For this reason, I believe the US military has gone with satellite navigation, so the position of each plane can be tracked by satellite and reported back to the various landing fields. Is this correct ? Are there plans to do this for commercial flights, as well ? StuRat (talk) 18:33, 18 February 2011 (UTC)
- Unless the military changed drastically in the last 20 years, there is a hell of a lot of ground-based radar data being used. My job was radar controller maintenance. The curvature of the Earth is handled by elevation. For example, I had to go to Norway (in February) to work on a radar positioned near the top of a mountain. Sure - a satellite may be able to monitor traffic around northern Norway, but having ground-based radar with a satellite uplink works better. -- kainaw™ 18:45, 18 February 2011 (UTC)
- (ec) The Tower, which I presume you mean the local air traffic control at an airfield, they are only interested in aircraft they can see out of the window and within ten or twenty miles from an airfield so radar being blocked by curvature is not normally a problem. The air traffic control centres need a bigger picture of what is going on and they can are normally be fed with radar images from different places some may be hundreds of miles from where the operator is stationed, think of it like an internet of radar images linked to different ATC towers and control centres. As to using satellites - have a read of Automatic dependent surveillance-broadcast not so much as the satellites tracking the aircraft they just use a system like the GPS/Sat Nav in you car and broadcast the position by radio. MilborneOne (talk) 18:55, 18 February 2011 (UTC)
- But how do the air traffic control centers get their data ? It can't be just the sum of all radar returns, since they won't work on flights across oceans. I suspect that they rely on info broadcast from the airplanes, but that could be missing or unreliable, in the case of an electrical failure or intentional incorrect transmissions, say from a terrorist-controlled airplane. StuRat (talk) 19:04, 18 February 2011 (UTC)
- My understanding is that oceanic flights are not tracked by ATC in real time. They are assigned a route when they leave one continent's coverage area, and are supposed to stick to it as best they can, or deport deviations by HF radio. (See procedural control]). TCAS still works to prevent mid-air collisions over the oceans; it does not depend on ground support (but uses some of the same onboard equipment as ATC uses for radar tracking). –Henning Makholm (talk) 16:08, 19 February 2011 (UTC)
- Most civilian aircraft in the United States (and much of the rest of the world) are tracked using Airport Surveillance RADAR. The newest model is ASR-11 and most civilian units are commercially sold by Raytheon Surveillance Systems, and then operated by local air traffic controllers and airports under contract to the FAA. The system works pretty well, and coverage is pretty dense, and the technology fits the organizational model currently in use to manage air traffic (which is as much a procedural challenge as a technology problem). Converting to a satellite-based system is surely possible - it's just expensive and unnecessary. The FAA's current "next big thing" for tracking civil air traffic is digital ASR: see this FAA press-release website. (After edit-conflict): civil RADARs are far more vulnerable to "spoofing" than military systems; they rely on squawk codes that aircraft voluntarily reports for such important data as elevation and aircraft status (plus "unimportant" metadata such as airline and flight number). In any case, this is not considered a serious threat to air defense, or else civil RADARs would be replaced by military-capable RADARs that have electronic countermeasures to make squawk spoofing and airborne electronic RADAR evasion more difficult. Finally, I refer you to Lincoln Laboratory Air Traffic Control Systems, an FFRDC funded by the U.S. Department of Defense and the FAA; this "think-tank" of aviation, electronics, and policy experts consider all of the sorts of issues that you are bringing up, and evaluate strategic technology and policy requirements for the national air traffic control network. In the same way that we have an article on everything, the Feds have an agency for everything. Nimur (talk) 19:16, 18 February 2011 (UTC)
Mental illness and responsibility of other for own thoughts and feeling
Is the exaggerated and persistent belief that others are responsibility for your own thoughts and feeling a common component of some mental illnesses? — Preceding unsigned comment added by Wikiweek (talk • contribs)
- I will provide almost the exact same answer as I provided to your earlier question. From one single "possible symptom", it is not possible for us, or even a trained professional, to make a judgement call about whether a person has a mental health issue. Correctly interpreting the results of a psychological screening or therapy interview is very difficult. That is why a psychiatrist is a trained medical doctor who has undergone several years of schooling, technical training, apprenticeship, and residency. A psychiatrist is able to meaningfully interpret the entirety of a person's circumstance and situation, not just the response to a single question. "Short questionnaires" should not be considered conclusive in any way; at best, they may help guide a trained professional by providing a wide set of indicators; but they are not a substitute for professional diagnosis. The reference desk will not provide medical advice, including psychiatric advice; as part of this, we will not be able to provide a concrete answer to your question, because it would constitute a diagnosis and we will not perform psychiatric diagnosis here. You can read our articles on mental health, and draw your own conclusions; and if you need assistance with diagnosis, you should seek a trained and licensed professional. Nimur (talk) 20:56, 18 February 2011 (UTC)
- We can't diagnose you here, nor can we offer you any form of treatment. However, that sounds similar to "institutional think"....Then again, mental illness itself may be a delusion per Thomas Szasz's Ideology and Insanity: Essays on the Psychiatric Dehumanization of Man.Smallman12q (talk) 21:41, 18 February 2011 (UTC)
- Actually there is a pretty straightforward answer. The belief that thoughts and sensations are being inserted into your head by external entities is a common feature of paranoid schizophrenia. Note that this doesn't just mean holding others responsible, it means believing that some entity is physically broadcasting thoughts or voices into your head. Merely blaming others for one's problems is not particularly meaningful. (Note: I don't see the question as asking for a diagnosis.) Looie496 (talk) 22:25, 18 February 2011 (UTC)
Tappan zee bridge cost
In this WSJ article on the Tappan Zee Bridge, it states that the bridge cost $640 million to build in today's dollars in the 1950's. The article also states "And the state has a team of financiers scrambling to find the $8.3 billion needed to replace it as a car-only structure without adding bus lanes or a train line and more than $16 billion with them."
Why would it cost more than 10x to build replace the bridge today than it did in 1950 (or did the WSJ do its math wrong?)Smallman12q (talk) 21:47, 18 February 2011 (UTC)
- The cost of building materials and the cost of laborers has gone up faster than inflation. Converting to "today's dollars" adjusts for inflation, but inflation tracks money supply relative to number of people. The cost to build something does not necessarily match. Another expense is dealing with rerouting all the traffic and people in the area of the construction. Especially in NY this could be a significant expense. Ariel. (talk) 22:33, 18 February 2011 (UTC)
- It's likely the new bridge would be designed to carry more traffic than the old bridge; a bigger bridge costs more money. And while I'm speculating, it may also be the case that a new bridge would need to be built to stricter standards than the old bridge was. —Bkell (talk) 00:54, 19 February 2011 (UTC)
- Adding weight to that interpretation is a passage from our article: "it was constructed during material shortages during the Korean War and designed to last only 50 years.... The collapse of the I-35W Mississippi River bridge in Minnesota on August 1, 2007 has renewed concerns about the bridge's structural integrity." TenOfAllTrades(talk) 14:29, 19 February 2011 (UTC)
- In addition to the original very lightweight design I noted above, the proposed replacement has a significantly expanded deck. The original bridge has seven lanes, and connects to at least eight lanes of highway at each end. The further lack of shoulders means that access for emergency vehicles can be an issue, and that any traffic problems rapidly snarl up flow along the entire bridge. The proposed design has ten lanes of automobile traffic, plus shoulders, plus dedicated space for pedestrian and bicycle traffic ([21]). TenOfAllTrades(talk) 14:48, 19 February 2011 (UTC)
Solar mass loss and orbits
How did scientists estimate Sun to last for about 4.5 billion years? The far I know is a loss of about 1% of solar mass through radiation which if calculated linearly should result in about 79 billion years given the current hydrogen and helium figures. On the other hand, what was the expected Earth's orbit when it began to form?--Email4mobile (talk) 22:10, 18 February 2011 (UTC)
- It's not linear in the slightest (over the entire life). See Stellar evolution for some discussions, but no numbers that I could see. In the current stage (of our sun) it's linear, but it eventually reaches an exhaustion point after which it changes dramatically. Even though it has tons of mass left it's not able to fuse it like it did before. And finally even if it was linear not all the mass gets converted to energy, only the mass deficit between hydrogen and helium gets converted - but the mass of the helium stays (ignoring the fact that it will fuse helium too). Rerun your calculations using the mass deficit instead of the total mass and see what you get. Ariel. (talk) 22:39, 18 February 2011 (UTC)
- A not-too-technical overview is provided here, at "Ask An Astronomer" from Cornell University. The lifetime of the sun is "estimated by assuming that the sun will "die" when it runs out of energy to keep it shining." The amount of available energy is estimated from knowledge of nuclear fusion; and the "energy consumption rate" is estimated from observations of the sun's energy output (how "brightly" it is shining). Nimur (talk) 22:52, 18 February 2011 (UTC)
Note though that the dating of the origin of the Sun doesn't rely on these things. The most precise figures are based on measurements of decay of radioactive elements in meteorites, which almost uniformly date to 4.5-4.6 billion years old, and which are believed on the basis of theoretical considerations to have formed within the first few million years of the solar system. Looie496 (talk) 23:53, 18 February 2011 (UTC)
- And during those 4.6bn years, the Sun will continue burning more-or-less the same as it does now. One quite-interesting problem of the late-19th century: by this time, Darwin was roughly accepted by the scientific community and gradualist geology suggested the Earth was 4bn years old. However, astrophysicists disagreed in theory, and came up with a maximum age of the Earth at 100,000 years. This is by no means Young-Earth Creationism, but the controversy was not without its theological side. The problem was that until the 1920s, everything in the universe was believed to revolve around the brilliant science of statistical mechanics, which can be derived entirely from a priori principles - that is, you don't even need to know what warmth looks like to know what temperature is. So by an ordinary thermodynamic engine with the size and temperature and fuel mass of the Sun, it could not even last a million years before burning out by even 100%-efficient engines. Then we discovered radioactive decay, the quantum mysteries of matter, and the nature of the atomic nucleus, and suddenly we had an entirely new engine in the universe: the nuclear forces. (There's a quote, probably by someone on the Bethe-Critchfield Nobel team for making fusion, when he's out with a companion at night: She said "Look at how pretty the stars shine!" He said "Yes, and right now I am the only man in the world who knows why they shine." According to the story, this was not enough to get her into bed. Amateur.) SamuelRiv (talk) 22:03, 19 February 2011 (UTC)
Effects to an Earth Human upon entering a parallel space-time continuum via a higher space-time continuum.
If an Earth Human would step through an inter-dimensional portal where the new space-time continuum was controlled by a Time "arrow" (as posited by the late Dr. Hawking, in his most famous book) that was pointing in the reverse direction to the Earth's time arrow, would the Earth Human immediately meld with the new time arrow, atom per atom, so to speak; in which case he would continue to age, even when he returned, he would return more aged, but to an earlier Earth time period?
K. McIntire-Tregoning, concert composer, February 18, 2011
(NOTE: copyrights suspended for this transmission with Wikipedia.) 189.173.210.245 (talk) 23:25, 18 February 2011 (UTC)
- Mind me asking who is that late Dr Hawking? Stephen Hawking is still alive. Dauto (talk) 02:29, 19 February 2011 (UTC)
- Anyways, any, any, ANY so called "time machine", i.e. a device/portal/spacetime construction/anything which takes you backwards in time will be destroyed by vacuum fluctuations within 10-43 seconds (The Planck-Wheeler time). So no, even if the question had some meaning, it wouldn't work. ManishEarthTalk • Stalk 10:22, 19 February 2011 (UTC)
- Could you provide some reference for that? Problems with time travel are usually described in terms of the Einstein Field Equations in General Relavity (and any solutions thereto requiring regions with negative energy density, which seem to be impossible). I've never heard of quantum mechanics and the Planck time being involved. --Tango (talk) 18:21, 19 February 2011 (UTC)
- If doesn't really make sense to talk about the arrow of time being in a different direction in one universe than in another. How would you compare them? Discussions about the arrow of time usually revolve around making sense of the relationships between different arrows of time within one universe: 1) We remember the past but we don't remember the future (the psychological arrow of time), 2) the universe is denser in the past than the future (the cosmological arrow of time) and 3) entropy (disorder) is lower in the past than in the future (the thermodynamic arrow of time). Some people describe other arrows of time, but they can usually be shown very easy to be equivalent to one of those three. What Stephen Hawking worked on (among many other things) was trying to work out whether it is concidence that, for example, we remember times of lower entropy and not ones of higher entropy or if the universe has to work that way (in this case, he realised that the act of storing memories in the brain by necessity increases entropy, so the arrows have to point in the relative directions that they do). Trying to compare arrows of time between universes makes no sense. The entire concept of different universes is difficult enough to make sense of. --Tango (talk) 18:21, 19 February 2011 (UTC)
February 19
Explosively formed penetrator efficiency
About how efficient would this be in terms of transferring chemical energy in the explosive to kinetic energy imparted to the penetrator? ScienceApe (talk) 03:18, 19 February 2011 (UTC)
- The chemical energy is converted into 1) kinetic energy (ie. that due to the projectile's initial velocity after the explosion) and 2) the energy necessary to "re-shape" the material of which the projectile consists. How much energy is required for that second process can be complicated, and I haven't been able to find a very good generally-applicable answer for you, but see eg. Plasticity (physics). You may also be interested in our Shaped charge article. WikiDao ☯ 14:32, 19 February 2011 (UTC)
Relativity
Is it possible for a bubble of spacetime to exist within this universe under the following conditions:
- There are two observers, observer A is located within this universe, B is located in the bubble.
- A and B agrees on the temporal vector however, they do not agree on the dimension vectors - each observer claims that the other's space is inversed, left is right, up is down.
Plasmic Physics (talk) 04:12, 19 February 2011 (UTC)
- Your question as it stands isn't well defined. People can "claim" whatever they want; you need to specify some kind of physical reversal effect that everyone agrees took place. For example, is there a spacetime manifold in which you can leave on a voyage and come back mirror-reflected (compared to someone who stayed home)? The answer to that is yes, since space could be shaped like the 3D analogue of a Klein bottle. But this is not really a relativistic question. General relativity doesn't say anything about what spacetime shapes are allowed. The Standard Model of particle physics is not mirror-symmetric, which suggests that such spacetimes aren't possible. -- BenRG (talk) 04:59, 19 February 2011 (UTC)
I'm not attempting to discuss the topology of the universe, only of a completely enclosed domain within the universe. The surface of the bubble is such that both observers may freely transit the bubble. There is no way for either observer to determine their absolute dimentional directionality, as there is no absolute reference frame. All they can say, is that the opposite observer's dimensionality is reversed. Plasmic Physics (talk) 05:29, 19 February 2011 (UTC)
- (EC) It's not clear what you mean by "a bubble of spacetime ... within this universe". Our universe by definition is a single connected spacetime manifold; see Universe#Definition as connected space-time. If there was a bubble of spacetime that's different from our normal universe, it would be a different, disconnected universe. The laws of physics in our universe have CPT symmetry. Parity alone is not conserved, as weak interactions violate parity, so a left vs. right transformation alone, or an up vs. down transformation alone, makes a difference as to our laws of physics. Ideas have been floated that perhaps there exist other universes that have different laws of physics, or at least have different values for physical constants, but such ideas are of course highly speculative, and are probably unfalsifiable. Red Act (talk) 05:34, 19 February 2011 (UTC)
- I still don't think the question makes sense. In a lot of relativity textbooks there are poor imitations of Einstein's thought experiments with wording like "observer A claims [something], while observer B claims [something different]". The physical content of these examples is no different from "observer A claims that she is larger than observer B, while observer B claims that it is he who is larger than observer A." The description of the relative sizes of objects in these people's visual fields is correct, but the use of the word "claims" is inappropriate, since it suggests that these observers reject the reality of the other's perceptions and are unable to understand the difference between the perception and the objective world. There are people like that in real life, admittedly, but I think the people in thought experiments should be competent scientists. If you're using the word "claims" in that way, then what you're really asking is whether it's possible for two people to each see the other as mirror-imaged or as upside-down. The answer is yes, since you can just place a mirror or lens at an appropriate spot between them. I know this isn't a very satisfying answer, but it may be the only correct answer. -- BenRG (talk) 06:47, 19 February 2011 (UTC)
That is exactly what I'm asking, but I'm asking whether it is at all possible without the use of a lense or mirror like object, a situation where the observation of parity reversal is independent of the relative location of either observer within their dommain with respect to the bubble's surface. The effect is only observed for lenses within a limited range for a lense or mirror. Plasmic Physics (talk) 08:16, 19 February 2011 (UTC)
- Oh, I get it. I'm sorry about the confusion. As a question in flat-space optics I think the answer is provably no, but I'm not sure. As a question in general relativity I'm not sure it makes sense, because whatever gravitational effects apply to light crossing the surface will also apply to matter crossing it. Maybe you could get around that with some crazy spacetime where lightlike geodesics get flipped around and timelike geodesics don't, but it seems dubious. -- BenRG (talk) 09:43, 19 February 2011 (UTC)
- Maybe if the bubble was a spherical "trench" in spacetime (circular trench in 2D spacetime)? It could quite easily refract the rays of light.
So, the solution is purely an optical manipulation. A circular trench wouldn't cause these effects, the light would be refracted around the bubble turning it invisible, rather like a metamaterial. Plasmic Physics (talk) 14:04, 19 February 2011 (UTC)
- You could twist spacetime, like it gets twisted around a spinning black hole, but you'd need a lot of twist. ManishEarthTalk • Stalk 16:10, 19 February 2011 (UTC)
It's not clear what the exact condition you're looking for. If you place two persons in Quito and Singapore, they will disagree about the up/down and east/west directions, but still agree about north and south. If you have two observers disagreeing about the signs of all three spatial dimensions, their disagreement will have to be purely conventional in the sense that you cannot turn one's experience continuously into the other's without passing through a degenerate state. (The transformation from one's coordinates to the other's will initially have negative determinant, and the determinant cannot pass through 0 while still denoting something physically meaningful). –Henning Makholm (talk) 17:56, 19 February 2011 (UTC)
Carbon black
wtf is carbon black — Preceding unsigned comment added by Tomjohnson357 (talk • contribs) 05:15, 19 February 2011
- Carbon black: "Carbon black is a material produced by the incomplete combustion of heavy petroleum products such as FCC tar, coal tar, ethylene cracking tar, and a small amount from vegetable oil....used as a pigment and reinforcement in rubber and plastic products" HiLo48 (talk) 05:25, 19 February 2011 (UTC)
- The original ("informal") format of this question was restored, see the discussion and the relevant edit diff. Nimur (talk) 19:00, 19 February 2011 (UTC)
height (and body size, really) variance in animals besides humans -- or the lack thereof?
The article human height places responsibility for the great variability in height across Homo sapiens largely in the hands of health and genetics. Now, I am a birdwatcher, and it occurred to me the other day that I could not recall ever seeing a crow or pigeon that was a head above his peers. Observing the Eurasian Tree Sparrow, of which we have many in China, one can note slight variations in body weight (particularly towards the end of winter) but again - no feathered Yao Ming or I suppose Deng Xiaoping stands out among the crowd. Are humans unusual among animals with respect to their readily observable variance in body length? I am excluding, of course, those species which never cease to grow - we must confine ourselves to those that reach a defined adult form and cease growing. The Masked Booby (talk) 09:33, 19 February 2011 (UTC)
- I thought in many mammals where size advantage in competition for a mate knocked up against genetic disadvantages of being too big there was reasonable variability. Certainly there seems to be size difference between adult male deer, and between adult hares and adult rabbits. Presumably the rest of the animal kingdom too but I can only speak for the ones I see often. --BozMo talk 10:06, 19 February 2011 (UTC)
- Consider the domestic dog, which ranges in size from the chihuahua to the Alaskan Malamute or Irish Wolfhound. This variance is the product of years of selective breeding. This, I think, is the key to your question: among domestic breeds, there is a great variation in size, but their wild equivalents are all pretty much the same. --TammyMoet (talk) 10:12, 19 February 2011 (UTC)
Dog mortality and longevity
Looking at the links above relating to the longevity of various dog breeds, I was surprised that they may on average only live 6-7 years and then die of cancer. 1) Why do dogs get cancer so early compared with humans? 2) Which dog breeds live longest, and least? 3) Is there any rule which predicts dog longevity from the breed size, etc? Thanks 92.15.16.146 (talk) 13:09, 19 February 2011 (UTC)
- WP:OR generality here but small dogs often live longer than large dogs. Oh, and if you want a source with some data, here ya go. Dismas|(talk) 15:03, 19 February 2011 (UTC)
- This is an area where numbers are varying rapidly due to social changes, and not evenly. People in advanced western nations these days seek more veterinary help for their pets than they would have 50 years ago. This makes a big difference. Some more very personal OR: breeds like Beagles, which are prone to escaping and demonstrating little road sense, are being penned and restrained more successfully these days, meaning that they are are overcoming their genetic predisposition to being run over. So that breed is experiencing a bigger improvement in longevity than some others. HiLo48 (talk) 15:21, 19 February 2011 (UTC)
- OR here, but mongrels seem to live much longer and healthier lives than highly bred animals. DuncanHill (talk) 20:14, 19 February 2011 (UTC)
- This is an area where numbers are varying rapidly due to social changes, and not evenly. People in advanced western nations these days seek more veterinary help for their pets than they would have 50 years ago. This makes a big difference. Some more very personal OR: breeds like Beagles, which are prone to escaping and demonstrating little road sense, are being penned and restrained more successfully these days, meaning that they are are overcoming their genetic predisposition to being run over. So that breed is experiencing a bigger improvement in longevity than some others. HiLo48 (talk) 15:21, 19 February 2011 (UTC)
Milk of Magnesia/Mike and Ikes
I recently discovered (about 5 minutes ago) that the candy Mike and Ikes contain magnesium hydroxide in trace quantities. Isn't that the main component of milk of magnesia, an antacid/laxative? Why is it there? Finalius (Say what?) 13:33, 19 February 2011 (UTC)
- I would imagine that the concentration in the candy will be very low. Magnesium hydroxide can fill a number of roles in foods and supplements, including "Drying Agent, pH Buffer, Antacid, Color Retention Agent". I suspect that its purpose here is to act as a drying agent, to keep the individual candies from getting tacky and sticking together. TenOfAllTrades(talk) 15:05, 19 February 2011 (UTC)
- (ec)According to WHO/FAO, it's an additive used as an "Acidity regulator [and] Colour retention agent".[22] As with many, many chemicals, a megadose can have very different effects than a small amount mixed with some other material. Also, we don't know (and I bet Just Born won't tell us) exactly how it's used...in combination with other ingredients, it could react to form different chemicals with different properties rather than just being diluted to low levels. DMacks (talk) 15:09, 19 February 2011 (UTC)
Why is the sun not purple or why is hydrogen plasma not yellow?
According to the article, hydrogen, the plasma state is purple, but the sun is yellowish, but it's made primarily out of hydrogen plasma. I know that black body radiation means that it should glow yellow according to that temperature, but then shouldn't hydrogen plasma glow yellowish too then if it's the same temperature? ScienceApe (talk) 16:28, 19 February 2011 (UTC)
- What makes you think the purple discharge shown in the hydrogen article is at the same temperature as the Sun('s photosphere)? –Henning Makholm (talk) 18:00, 19 February 2011 (UTC)
- The image of "purple" hydrogen is dominated by spectral emissions, not thermal radiation. Hydrogen plasma can be purple because one of the characteristic Hydrogen electron transitions (specifically, the 6→2 transition is purplish. In this particular lab-setup, that energy level has been stimulated.
- The sun's spectrum is dominated by thermal blackbody radiation - that is, it is not spectral emission lines. Our sunlight article has a whole section on solar spectrum composition explaining the details. There are a few "peaks" and "notches" superimposed on top of the ideal black-body curve, due to photochemical absorption (i.e. spectral absorption) and atomic emission spectra inside the sun. The spectrum received at Earth's surface is further "notched" by spectral absorption of chemicals in the Earth's atmosphere (in the visible spectrum, most absorption lines are caused by water, CO2, and a few ions). Nimur (talk) 19:10, 19 February 2011 (UTC)
- Well I assumed that plasma is still pretty hot. I know you can get cold plasmas but I just assumed that plasma was hot. I dono, maybe it's not hot. Could a black dwarf look purple in that case? ScienceApe (talk) 19:32, 19 February 2011 (UTC)
- It's a question of optical depth; while the plasma emits more at the spectral lines of hydrogen, it also absorbs more in these parts of the spectrum. You can imagine a piece of plasma absorbing the incoming light at the spectral lines strongly, but absorbing far less (in relative terms) of the light between the spectral lines. This piece of plasma will then emit strongly at the spectral lines and weakly between the spectral lines. The resulting light will have a lower ratio of light intensity at the spectral lines and between the spectral lines than the incoming light. In other words, you see deeper into the Sun at frequencies which are not at the spectral lines of hydrogen, and if you would put many such purple laboratory plasma vessels behind each other, they probably wouldn't look purple anymore. Icek (talk) 20:34, 19 February 2011 (UTC)
- You do get purple light from optically thin HII regions in the interstellar medium. The purple line is actually the red Hα line (6565 Angstrom), that is the 3-2 transition (6-2 is Hδ which is weaker and lies at the blue/violet end of the visible spectral range). These lines arise when an electron and a proton in an ionised hydrogen gas recombine to form a hydrogen atom; the electron cascades down to the ground level (n=1) and in the course of that cascade, many electrons go through n=3 and n=2, making Hα one of the strongest emission lines. In an optically thin gas, the photons emitted that way escape immediately. In an optically thick gas, they are reabsorbed very quickly. In the sun, the radiation originates deep in the core of the sun, and while diffusing outwards is scattered, absorbed and reemitted many many times, leading to thermalisation of the radiation and thus a black-body spectrum. This spectrum is modified by the outermost layer of the sun, just when the radiation leaves the sun. This modification takes the form of absorption lines, not emission lines. --Wrongfilter (talk) 20:46, 19 February 2011 (UTC)
- My description might have been misleading; the reason for the lines being absorption is that the outermost layers are the coolest layers, therefore in these layers it will more often happen that an atom absorbs radiation than that it emits radiation. That's of course not the case with many many laboratory plasma vessels; the lines would vanish alltogether if there is a sufficient number of them. Icek (talk) 21:00, 19 February 2011 (UTC)
Common hazel (Corylus avellana)
Can the climate in the midwestern US support common hazel (Corylus avellana)? --75.15.161.185 (talk) 19:56, 19 February 2011 (UTC)













